Improved MECP2 Gene Therapy Extends the Survival of MeCP2-Null Mice without Apparent Toxicity after Intracisternal Delivery
- PMID: 28497072
- PMCID: PMC5424572
- DOI: 10.1016/j.omtm.2017.04.006
Improved MECP2 Gene Therapy Extends the Survival of MeCP2-Null Mice without Apparent Toxicity after Intracisternal Delivery
Abstract
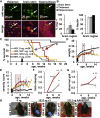
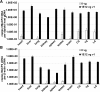
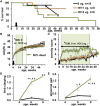
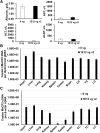
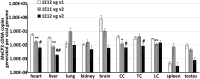
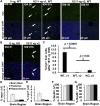
Intravenous administration of adeno-associated virus serotype 9 (AAV9)/hMECP2 has been shown to extend the lifespan of Mecp2-/y mice, but this delivery route induces liver toxicity in wild-type (WT) mice. To reduce peripheral transgene expression, we explored the safety and efficacy of AAV9/hMECP2 injected into the cisterna magna (ICM). AAV9/hMECP2 (1 × 1012 viral genomes [vg]; ICM) extended Mecp2-/y survival but aggravated hindlimb clasping and abnormal gait phenotypes. In WT mice, 1 × 1012 vg of AAV9/hMECP2 induced clasping and abnormal gait. A lower dose mitigated these adverse phenotypes but failed to extend survival of Mecp2-/y mice. Thus, ICM delivery of this vector is impractical as a treatment for Rett syndrome (RTT). To improve the safety of MeCP2 gene therapy, the gene expression cassette was modified to include more endogenous regulatory elements believed to modulate MeCP2 expression in vivo. In Mecp2-/y mice, ICM injection of the modified vector extended lifespan and was well tolerated by the liver but did not rescue RTT behavioral phenotypes. In WT mice, these same doses of the modified vector had no adverse effects on survival or neurological phenotypes. In summary, we identified limitations of the original vector and demonstrated that an improved vector design extends Mecp2-/y survival, without apparent toxicity.
Keywords: AAV; MeCP2; Mecp2−/y; Rett syndrome; cisterna magna; intrathecal; microRNA; viral vector.
Figures


References
-
- Amir R.E., Van den Veyver I.B., Wan M., Tran C.Q., Francke U., Zoghbi H.Y. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999;23:185–188. - PubMed
-
- Shahbazian M.D., Antalffy B., Armstrong D.L., Zoghbi H.Y. Insight into Rett syndrome: MeCP2 levels display tissue- and cell-specific differences and correlate with neuronal maturation. Hum. Mol. Genet. 2002;11:115–124. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

