Accumbal Cholinergic Interneurons Differentially Influence Motivation Related to Satiety Signaling
- PMID: 28497110
- PMCID: PMC5422920
- DOI: 10.1523/ENEURO.0328-16.2017
Accumbal Cholinergic Interneurons Differentially Influence Motivation Related to Satiety Signaling
Abstract
Satiety, rather than all or none, can instead be viewed as a cumulative decrease in the drive to eat that develops over the course of a meal. The nucleus accumbens (NAc) is known to play a critical role in this type of value reappraisal, but the underlying circuits that influence such processes are unclear. Although NAc cholinergic interneurons (CINs) comprise only a small proportion of NAc neurons, their local impact on reward-based processes provides a candidate cell population for investigating the neural underpinnings of satiety. The present research therefore aimed to determine the role of NAc-CINs in motivation for food reinforcers in relation to satiety signaling. Through bidirectional control of CIN activity in mice, we show that when motivated by food restriction, increasing CIN activity led to a reduction in palatable food consumption while reducing CIN excitability enhanced food intake. These activity-dependent changes developed only late in the session and were unlikely to be driven by the innate reinforcer strength, suggesting that CIN modulation was instead impacting the cumulative change in motivation underlying satiety signaling. We propose that on a circuit level, an overall increase in inhibitory tone onto NAc output neurons played a role in the behavioral results, as activating NAc-CINs led to an inhibition of medium spiny neurons that was dependent on nicotinic receptor activation. Our results reveal an important role for NAc-CINs in controlling motivation for food intake and additionally provide a circuit-level framework for investigating the endogenous cholinergic circuits that signal satiety.
Conflict of interest statement
Authors report no conflict of interest.
Figures

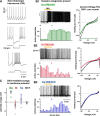
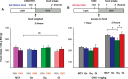


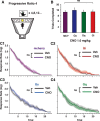

References
-
- Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, McNamara JO (2009) Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 63:27–39. 10.1016/j.neuron.2009.06.014 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
