Modulation of basal cell fate during productive and transforming HPV-16 infection is mediated by progressive E6-driven depletion of Notch
- PMID: 28497579
- PMCID: PMC5601300
- DOI: 10.1002/path.4917
Modulation of basal cell fate during productive and transforming HPV-16 infection is mediated by progressive E6-driven depletion of Notch
Abstract
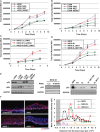
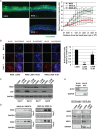
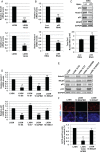
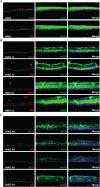
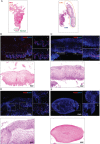
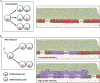
In stratified epithelia such as the epidermis, homeostasis is maintained by the proliferation of cells in the lower epithelial layers and the concomitant loss of differentiated cells from the epithelial surface. These differentiating keratinocytes progressively stratify and form a self-regenerating multi-layered barrier that protects the underlying dermis. In such tissue, the continual loss and replacement of differentiated cells also limits the accumulation of oncogenic mutations within the tissue. Inactivating mutations in key driver genes, such as TP53 and NOTCH1, reduce the proportion of differentiating cells allowing for the long-term persistence of expanding mutant clones in the tissue. Here we show that through the expression of E6, HPV-16 prevents the early fate commitment of human keratinocytes towards differentiation and confers a strong growth advantage to human keratinocytes. When E6 is expressed either alone or with E7, it promotes keratinocyte proliferation at high cell densities, through the combined inactivation of p53 and Notch1. In organotypic raft culture, the activity of E6 is restricted to the basal layer of the epithelium and is enhanced during the progression from productive to abortive or transforming HPV-16 infection. Consistent with this, the expression of p53 and cleaved Notch1 becomes progressively more disrupted, and is associated with increased basal cell density and reduced commitment to differentiation. The expression of cleaved Notch1 is similarly disrupted also in HPV-16-positive cervical lesions, depending on neoplastic grade. When taken together, these data depict an important role of high-risk E6 in promoting the persistence of infected keratinocytes in the basal and parabasal layers through the inactivation of gene products that are commonly mutated in non-HPV-associated neoplastic squamous epithelia. © 2017 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of Pathological Society of Great Britain and Ireland.
Keywords: E6; HPV; NICD; Notch; differentiation; p53.
© 2017 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of Pathological Society of Great Britain and Ireland.
Figures
References
-
- de Villiers EM, Fauquet C, Broker TR, et al Classification of papillomaviruses. Virology 2004; 324: 17‐27. - PubMed
-
- zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer 2002; 2: 342–350. - PubMed
-
- Doorbar J, Quint W, Banks L, et al The biology and life‐cycle of human papillomaviruses. Vaccine 2012; 30 (suppl 5): F55‐F70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

