Broad Th2 neutralization and anti-inflammatory action of pentosan polysulfate sodium in experimental allergic rhinitis
- PMID: 28497614
- PMCID: PMC5569365
- DOI: 10.1002/iid3.164
Broad Th2 neutralization and anti-inflammatory action of pentosan polysulfate sodium in experimental allergic rhinitis
Abstract
Background: Th2 cytokines like interleukin-4, -5, and -13 are regarded as important drivers of the immunopathology underlying allergic rhinitis (AR) and asthma. The present study explores the capacity of pentosan polysulfate sodium (PPS), a semi-synthetic heparin-like macromolecular carbohydrate, to bind Th2 cytokines and exert biological neutralization in vitro, as well as anti-inflammatory actions in vivo.
Methodology: The capacity of PPS to bind recombinant Th2 cytokines was tested with surface plasmon resonance (SPR) technology and biological Th2 neutralization was assessed by Th2-dependent proliferation assays. The in vivo anti-inflammatory action of PPS was studied using a validated Guinea-pig model of AR.
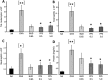
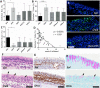
Results: Binding studies revealed a strong and specific binding of PPS to IL-4, IL-5, and IL-13 with IC values suggesting as stronger cytokine binding than for heparin. Cytokine binding translated to a biological neutralization as PPS dose dependently inhibited Th2-dependent cell proliferation. Topical administration of PPS 30 min prior to nasal allergen challenge of sensitized animals significantly reduced late phase plasma extravasation, luminal influx of eosinophils, neutrophils, and total lavage leukocytes. Similar, albeit not statistically secured, effects were found for tissue leukocytes and mucus hyper-secretion. The anti-inflammatory effects of PPS compared favorably with established topical nasal steroid treatment.
Conclusion: This study points out PPS as a potent Th2 cytokine-binding molecule with biological neutralization capacity and broad anti-inflammatory effects in vivo. As such PPS fulfills the role as a potential candidate molecule for the treatment of AR and further studies of clinical efficacy seems highly warranted.
Keywords: Allergy; Th2 cytokines; interleukin-13; interleukin-4; interleukin-5; pentosan polyphosphate sodium.
© 2017 The Authors. Immunity, Inflammation and Disease Published by John Wiley & Sons Ltd.
Figures


Similar articles
-
Hydrogen-Rich Saline Ameliorates Allergic Rhinitis by Reversing the Imbalance of Th1/Th2 and Up-Regulation of CD4+CD25+Foxp3+Regulatory T Cells, Interleukin-10, and Membrane-Bound Transforming Growth Factor-β in Guinea Pigs.Inflammation. 2018 Feb;41(1):81-92. doi: 10.1007/s10753-017-0666-6. Inflammation. 2018. PMID: 28894978
-
Inhibition by pentosan polysulfate (PPS) of heparin-binding growth factors released from tumor cells and blockage by PPS of tumor growth in animals.J Natl Cancer Inst. 1992 Nov 18;84(22):1716-24. doi: 10.1093/jnci/84.22.1716. J Natl Cancer Inst. 1992. PMID: 1279186
-
Neutralization of interleukin-9 ameliorates symptoms of allergic rhinitis by reducing Th2, Th9, and Th17 responses and increasing the Treg response in a murine model.Oncotarget. 2017 Feb 28;8(9):14314-14324. doi: 10.18632/oncotarget.15177. Oncotarget. 2017. PMID: 28187441 Free PMC article.
-
The paradigm of cytokine networks in allergic airway inflammation.Curr Opin Allergy Clin Immunol. 2015 Feb;15(1):41-8. doi: 10.1097/ACI.0000000000000129. Curr Opin Allergy Clin Immunol. 2015. PMID: 25479317 Review.
-
IL-4/IL-13 axis as therapeutic targets in allergic rhinitis and asthma.PeerJ. 2022 May 30;10:e13444. doi: 10.7717/peerj.13444. eCollection 2022. PeerJ. 2022. PMID: 35663523 Free PMC article. Review.
Cited by
-
Mangiferin Alleviates Ovalbumin-Induced Allergic Rhinitis via Nrf2/HO-1/NF-κB Signaling Pathways.Int J Mol Sci. 2020 May 12;21(10):3415. doi: 10.3390/ijms21103415. Int J Mol Sci. 2020. PMID: 32408566 Free PMC article.
-
Pentosan Polysulfate Treatment of Mucopolysaccharidosis Type IIIA Mice.JIMD Rep. 2019;43:37-52. doi: 10.1007/8904_2018_96. Epub 2018 Apr 14. JIMD Rep. 2019. PMID: 29654542 Free PMC article.
-
Pentosan polysulfate sodium promotes redifferentiation to the original phenotype in micromass-cultured canine articular chondrocytes and exerts molecular weight-dependent effects.J Vet Med Sci. 2023 Jun 14;85(6):680-690. doi: 10.1292/jvms.22-0567. Epub 2023 May 8. J Vet Med Sci. 2023. PMID: 37150611 Free PMC article.
-
Topical Application of OJI-204 Alleviates Skin Dryness, Dry Skin-Induced Itch, and Mechanical Alloknesis.Biomedicines. 2025 Feb 21;13(3):556. doi: 10.3390/biomedicines13030556. Biomedicines. 2025. PMID: 40149533 Free PMC article.
-
Anti-inflammatory actions of Pentosan polysulfate sodium in a mouse model of influenza virus A/PR8/34-induced pulmonary inflammation.Front Immunol. 2023 Feb 9;14:1030879. doi: 10.3389/fimmu.2023.1030879. eCollection 2023. Front Immunol. 2023. PMID: 36845136 Free PMC article.
References
-
- Baena‐Cagnani, C. E. , Canonica G. W., Zaky Helal M., Gomez R. M., Compalati E., Zernotti M. E., Sanchez‐Borges M., Morato Castro F. F., Murrieta Aguttes M., Lopez‐Garcia A., et al. 2015. The international survey on the management of allergic rhinitis by physicians and patients (ISMAR). World Allergy Organ J. 8(1):10. - PMC - PubMed
-
- Eifan, A. O. , and Durham S. R.. 2016. Pathogenesis of rhinitis. Clin. Exp. Allergy. 46(9):1139–1151. - PubMed
-
- Howarth, P. H. , Salagean M., and Dokic D.. 2000. Allergic rhinitis: not purely a histamine‐related disease. Allergy. 55(Suppl 64):7–16. - PubMed
-
- May, R. D. , and Fung M.. 2015. Strategies targeting the IL‐4/IL‐13 axes in disease. Cytokine. 75(1):89–116. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

