Eukaryotic Translesion DNA Synthesis on the Leading and Lagging Strands: Unique Detours around the Same Obstacle
- PMID: 28497687
- PMCID: PMC5662946
- DOI: 10.1021/acs.chemrev.7b00046
Eukaryotic Translesion DNA Synthesis on the Leading and Lagging Strands: Unique Detours around the Same Obstacle
Abstract
During S-phase, minor DNA damage may be overcome by DNA damage tolerance (DDT) pathways that bypass such obstacles, postponing repair of the offending damage to complete the cell cycle and maintain cell survival. In translesion DNA synthesis (TLS), specialized DNA polymerases replicate the damaged DNA, allowing stringent DNA synthesis by a replicative polymerase to resume beyond the offending damage. Dysregulation of this DDT pathway in human cells leads to increased mutation rates that may contribute to the onset of cancer. Furthermore, TLS affords human cancer cells the ability to counteract chemotherapeutic agents that elicit cell death by damaging DNA in actively replicating cells. Currently, it is unclear how this critical pathway unfolds, in particular, where and when TLS occurs on each template strand. Given the semidiscontinuous nature of DNA replication, it is likely that TLS on the leading and lagging strand templates is unique for each strand. Since the discovery of DDT in the late 1960s, most studies on TLS in eukaryotes have focused on DNA lesions resulting from ultraviolet (UV) radiation exposure. In this review, we revisit these and other related studies to dissect the step-by-step intricacies of this complex process, provide our current understanding of TLS on leading and lagging strand templates, and propose testable hypotheses to gain further insights.
Figures

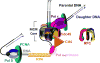

 ) on a lagging strand template. This model shown in cartoon form only displays pol α-primase on the lagging strand template for simplicity.
) on a lagging strand template. This model shown in cartoon form only displays pol α-primase on the lagging strand template for simplicity.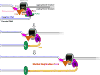
 ) within a leading strand template, DNA synthesis by pol ε abruptly stops but the CMG helicase remains intact and continues unwinding DNA, exposing long stretches of the leading strand template. During such uncoupling, RPA coats the exposed leading strand template and DNA synthesis continues on the undamaged lagging strand template as the replication fork progresses. Furthermore, non-replicating pol ε maintains contact with the CMG helicase and is carried downstream of the offending lesion while PCNA is left behind at the blocked P/T junction. Pol ε may also maintain contact with the blocked P/T junction upon uncoupling, forming ssDNA loop. However, such complexes are expected to be short-lived due to RPA●ssDNA interactions. (2) Uncoupled from leading strand DNA synthesis, the CMG helicase and, hence, lagging strand DNA synthesis eventually stall downstream of the offending damage, halting progression of the replication fork.
) within a leading strand template, DNA synthesis by pol ε abruptly stops but the CMG helicase remains intact and continues unwinding DNA, exposing long stretches of the leading strand template. During such uncoupling, RPA coats the exposed leading strand template and DNA synthesis continues on the undamaged lagging strand template as the replication fork progresses. Furthermore, non-replicating pol ε maintains contact with the CMG helicase and is carried downstream of the offending lesion while PCNA is left behind at the blocked P/T junction. Pol ε may also maintain contact with the blocked P/T junction upon uncoupling, forming ssDNA loop. However, such complexes are expected to be short-lived due to RPA●ssDNA interactions. (2) Uncoupled from leading strand DNA synthesis, the CMG helicase and, hence, lagging strand DNA synthesis eventually stall downstream of the offending damage, halting progression of the replication fork.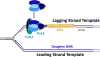
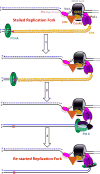
 ) encountered in a leading strand template leads to stalling of the replication fork downstream of the lesion (as depicted in Figure 5). TLS on a leading strand template can occur by one of two pathways. In “on the fly” TLS, bypass of the UV-induced lesion occurs before progression of the replication fork has been re-started through a re-priming event, as follows: 1) One or more TLS pols bypass the offending DNA lesion, extending the aborted primer terminus to an undamaged section of the leading strand template. 2) Pol δ faithfully extends the primer to the stalled CMG helicase where 3) the bound pol ε rapidly replaces pol δ on the leading strand template, re-starting progression of the stalled replication fork. In this scenario, replication fork re-start requires TLS. As an alternative to “on the fly” TLS, UV-induced lesions within a leading strand template may be bypassed by postreplicative gap-filling (Figure 8).
) encountered in a leading strand template leads to stalling of the replication fork downstream of the lesion (as depicted in Figure 5). TLS on a leading strand template can occur by one of two pathways. In “on the fly” TLS, bypass of the UV-induced lesion occurs before progression of the replication fork has been re-started through a re-priming event, as follows: 1) One or more TLS pols bypass the offending DNA lesion, extending the aborted primer terminus to an undamaged section of the leading strand template. 2) Pol δ faithfully extends the primer to the stalled CMG helicase where 3) the bound pol ε rapidly replaces pol δ on the leading strand template, re-starting progression of the stalled replication fork. In this scenario, replication fork re-start requires TLS. As an alternative to “on the fly” TLS, UV-induced lesions within a leading strand template may be bypassed by postreplicative gap-filling (Figure 8).
 ) encountered in a leading strand template leads to stalling of the replication fork downstream of the lesion (as depicted in Figure 5). In postreplicative gap-filling, bypass of the UV-induced lesion occurs after the replication fork has been re-started through a re-priming event, as follows. 1) PrimPol is recruited to the RPA-coated ssDNA downstream of a UV-induced lesion by a direct interaction with RPA. Once localized, PrimPol synthesizes a nascent primer at a random location downstream of the offending damage and a new PCNA ring is loaded onto the nascent 3′ hydroxyl terminus. This leaves behind an RPA-coated ssDNA gap (postreplicative gap) containing PCNA and the lesion. 2) Pol δ faithfully extends the nascent primer terminus to the stalled CMG helicase where 3) the bound pol ε rapidly replaces pol δ on the leading strand template, re-starting progression of the stalled replication fork. 4) Eventually, the offending DNA lesion within the postreplicative gap (shown) is bypassed by one or more TLS pols, extending the aborted primer terminus to an undamaged section of the leading strand template. 5) Pol δ “fills in” the remainder of the postreplicative gap and the 5′ RNA end of the downstream duplex region is removed as in Okazaki fragment processing/metabolism. 6) The resident PCNA is removed some time after the fully-extended primer terminus is ligated to the 5′ end of the downstream daughter DNA.
) encountered in a leading strand template leads to stalling of the replication fork downstream of the lesion (as depicted in Figure 5). In postreplicative gap-filling, bypass of the UV-induced lesion occurs after the replication fork has been re-started through a re-priming event, as follows. 1) PrimPol is recruited to the RPA-coated ssDNA downstream of a UV-induced lesion by a direct interaction with RPA. Once localized, PrimPol synthesizes a nascent primer at a random location downstream of the offending damage and a new PCNA ring is loaded onto the nascent 3′ hydroxyl terminus. This leaves behind an RPA-coated ssDNA gap (postreplicative gap) containing PCNA and the lesion. 2) Pol δ faithfully extends the nascent primer terminus to the stalled CMG helicase where 3) the bound pol ε rapidly replaces pol δ on the leading strand template, re-starting progression of the stalled replication fork. 4) Eventually, the offending DNA lesion within the postreplicative gap (shown) is bypassed by one or more TLS pols, extending the aborted primer terminus to an undamaged section of the leading strand template. 5) Pol δ “fills in” the remainder of the postreplicative gap and the 5′ RNA end of the downstream duplex region is removed as in Okazaki fragment processing/metabolism. 6) The resident PCNA is removed some time after the fully-extended primer terminus is ligated to the 5′ end of the downstream daughter DNA.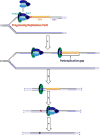
 ) within a lagging strand template but the replisome and, hence, the replication fork continue unimpeded (as detailed in Figure 6). Bypass of the offending damage occurs by postreplicative gap-filling as follows. 1) Pol α-primase performs “scheduled” synthesis of an RNA/DNA hybrid primer upstream (5′) of the offending damage on the exposed lagging strand template. PCNA residing at the blocked P/T junction remains and a pol δ holoenzyme is assembled on the nascent P/T junction with a new PCNA ring, allowing lagging strand DNA synthesis to resume and continue upstream of the offending DNA lesion. This prevents excessive exposure of the lagging strand template and generates a postreplicative gap less than or equal to the size of an Okazaki fragment (~100 – 250 nt) extending from the blocked P/T junction to the 5′ terminus of the downstream Okazaki fragment. As the replisome progresses in the absence of TLS, the replication fork moves further and further ahead of the postreplicative gap. 2) Eventually, one or more TLS pols bypass the offending DNA lesion within the postreplicative gap, extending the aborted primer terminus to an undamaged section of the lagging strand template. 3) Pol δ then “fills in” the remainder of the postreplicative gap and the 5′ RNA end of the downstream duplex region is removed as in Okazaki fragment processing/metabolism. 4) The resident PCNA is removed some time after the fully-extended primer terminus is ligated to the 5′ end of the downstream Okazaki fragment.
) within a lagging strand template but the replisome and, hence, the replication fork continue unimpeded (as detailed in Figure 6). Bypass of the offending damage occurs by postreplicative gap-filling as follows. 1) Pol α-primase performs “scheduled” synthesis of an RNA/DNA hybrid primer upstream (5′) of the offending damage on the exposed lagging strand template. PCNA residing at the blocked P/T junction remains and a pol δ holoenzyme is assembled on the nascent P/T junction with a new PCNA ring, allowing lagging strand DNA synthesis to resume and continue upstream of the offending DNA lesion. This prevents excessive exposure of the lagging strand template and generates a postreplicative gap less than or equal to the size of an Okazaki fragment (~100 – 250 nt) extending from the blocked P/T junction to the 5′ terminus of the downstream Okazaki fragment. As the replisome progresses in the absence of TLS, the replication fork moves further and further ahead of the postreplicative gap. 2) Eventually, one or more TLS pols bypass the offending DNA lesion within the postreplicative gap, extending the aborted primer terminus to an undamaged section of the lagging strand template. 3) Pol δ then “fills in” the remainder of the postreplicative gap and the 5′ RNA end of the downstream duplex region is removed as in Okazaki fragment processing/metabolism. 4) The resident PCNA is removed some time after the fully-extended primer terminus is ligated to the 5′ end of the downstream Okazaki fragment.
 ) is governed by three activities; 1) Enzymatic loading of PCNA onto the P/T junction; 2) Enzymatic unloading of PCNA from the P/T junction and; 3) diffusion of PCNA along either the nascent dsDNA or the adjacent ssDNA. If PCNA can vacate a blocked P/T junction, limiting PCNA rings will not be continuously re-loaded. However, diffusion of PCNA along DNA and enzyme-catalyzed unloading PCNA from DNA are prohibited during TLS, promoting retention of PCNA at blocked P/T junctions during S-phase.
) is governed by three activities; 1) Enzymatic loading of PCNA onto the P/T junction; 2) Enzymatic unloading of PCNA from the P/T junction and; 3) diffusion of PCNA along either the nascent dsDNA or the adjacent ssDNA. If PCNA can vacate a blocked P/T junction, limiting PCNA rings will not be continuously re-loaded. However, diffusion of PCNA along DNA and enzyme-catalyzed unloading PCNA from DNA are prohibited during TLS, promoting retention of PCNA at blocked P/T junctions during S-phase.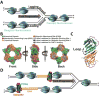
Similar articles
-
Polymerase Delta in Eukaryotes: How is It Transiently Exchanged with Specialized DNA Polymerases During Translesion DNA Synthesis?Curr Protein Pept Sci. 2018;19(8):790-804. doi: 10.2174/1389203719666180430155625. Curr Protein Pept Sci. 2018. PMID: 29708067 Review.
-
Characterization of human translesion DNA synthesis across a UV-induced DNA lesion.Elife. 2016 Oct 22;5:e19788. doi: 10.7554/eLife.19788. Elife. 2016. PMID: 27770570 Free PMC article.
-
Genetic control of translesion synthesis on leading and lagging DNA strands in plasmids derived from Epstein-Barr virus in human cells.mBio. 2012 Sep 11;3(5):e00271-12. doi: 10.1128/mBio.00271-12. Print 2012. mBio. 2012. PMID: 22967980 Free PMC article.
-
Implications of Translesion DNA Synthesis Polymerases on Genomic Stability and Human Health.Mol Cell Biol. 2023;43(8):401-425. doi: 10.1080/10985549.2023.2224199. Epub 2023 Jul 13. Mol Cell Biol. 2023. PMID: 37439479 Free PMC article.
-
DNA Damage Tolerance Pathways in Human Cells: A Potential Therapeutic Target.Front Oncol. 2022 Feb 7;11:822500. doi: 10.3389/fonc.2021.822500. eCollection 2021. Front Oncol. 2022. PMID: 35198436 Free PMC article. Review.
Cited by
-
Tracking of progressing human DNA polymerase δ holoenzymes reveals distributions of DNA lesion bypass activities.Nucleic Acids Res. 2022 Sep 23;50(17):9893-9908. doi: 10.1093/nar/gkac745. Nucleic Acids Res. 2022. PMID: 36107777 Free PMC article.
-
Inflammation-induced DNA damage, mutations and cancer.DNA Repair (Amst). 2019 Nov;83:102673. doi: 10.1016/j.dnarep.2019.102673. Epub 2019 Jul 25. DNA Repair (Amst). 2019. PMID: 31387777 Free PMC article. Review.
-
Replication protein A dynamically regulates monoubiquitination of proliferating cell nuclear antigen.J Biol Chem. 2019 Mar 29;294(13):5157-5168. doi: 10.1074/jbc.RA118.005297. Epub 2019 Jan 30. J Biol Chem. 2019. PMID: 30700555 Free PMC article.
-
Replisome structure suggests mechanism for continuous fork progression and post-replication repair.DNA Repair (Amst). 2019 Sep;81:102658. doi: 10.1016/j.dnarep.2019.102658. Epub 2019 Jul 8. DNA Repair (Amst). 2019. PMID: 31303546 Free PMC article. Review.
-
Ubiquitin and Ubiquitin-Like Proteins Are Essential Regulators of DNA Damage Bypass.Cancers (Basel). 2020 Oct 2;12(10):2848. doi: 10.3390/cancers12102848. Cancers (Basel). 2020. PMID: 33023096 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

