BLISS is a versatile and quantitative method for genome-wide profiling of DNA double-strand breaks
- PMID: 28497783
- PMCID: PMC5437291
- DOI: 10.1038/ncomms15058
BLISS is a versatile and quantitative method for genome-wide profiling of DNA double-strand breaks
Abstract
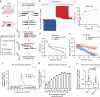
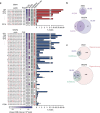
Precisely measuring the location and frequency of DNA double-strand breaks (DSBs) along the genome is instrumental to understanding genomic fragility, but current methods are limited in versatility, sensitivity or practicality. Here we present Breaks Labeling In Situ and Sequencing (BLISS), featuring the following: (1) direct labelling of DSBs in fixed cells or tissue sections on a solid surface; (2) low-input requirement by linear amplification of tagged DSBs by in vitro transcription; (3) quantification of DSBs through unique molecular identifiers; and (4) easy scalability and multiplexing. We apply BLISS to profile endogenous and exogenous DSBs in low-input samples of cancer cells, embryonic stem cells and liver tissue. We demonstrate the sensitivity of BLISS by assessing the genome-wide off-target activity of two CRISPR-associated RNA-guided endonucleases, Cas9 and Cpf1, observing that Cpf1 has higher specificity than Cas9. Our results establish BLISS as a versatile, sensitive and efficient method for genome-wide DSB mapping in many applications.
Conflict of interest statement
A patent application has been filed including work described in this publication. F.Z. is a cofounder of Editas Medicine and a scientific advisor for Editas Medicine and Horizon Discovery. The remaining authors declare no competing financial interests.
Figures

References
-
- Baudat F., Imai Y. & de Massy B. Meiotic recombination in mammals: localization and regulation. Nat. Rev. Genet. 14, 794–806 (2013). - PubMed
-
- Schatz D. G. & Swanson P. C. V(D)J Recombination: mechanisms of initiation. Annu. Rev. Genet. 45, 167–202 (2011). - PubMed
-
- Negrini S., Gorgoulis V. G. & Halazonetis T. D. Genomic instability--an evolving hallmark of cancer. Nat. Rev. Mol. Cell Biol. 11, 220–228 (2010). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

