Structural heterogeneity in the intrinsically disordered RNA polymerase II C-terminal domain
- PMID: 28497792
- PMCID: PMC5437306
- DOI: 10.1038/ncomms15231
Structural heterogeneity in the intrinsically disordered RNA polymerase II C-terminal domain
Abstract
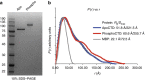
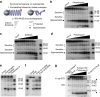
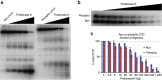
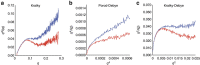
RNA polymerase II contains a repetitive, intrinsically disordered, C-terminal domain (CTD) composed of heptads of the consensus sequence YSPTSPS. The CTD is heavily phosphorylated and serves as a scaffold, interacting with factors involved in transcription initiation, elongation and termination, RNA processing and chromatin modification. Despite being a nexus of eukaryotic gene regulation, the structure of the CTD and the structural implications of phosphorylation are poorly understood. Here we present a biophysical and biochemical interrogation of the structure of the full length CTD of Drosophila melanogaster, which we conclude is a compact random coil. Surprisingly, we find that the repetitive CTD is structurally heterogeneous. Phosphorylation causes increases in radius, protein accessibility and stiffness, without disrupting local structural heterogeneity. Additionally, we show the human CTD is also structurally heterogeneous and able to substitute for the D. melanogaster CTD in supporting fly development to adulthood. This finding implicates conserved structural organization, not a precise array of heptad motifs, as important to CTD function.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Chapman R. D., Heidemann M., Hintermair C. & Eick D. Molecular evolution of the RNA polymerase II CTD. Trends Genet. 24, 289–296 (2008). - PubMed
-
- Eick D. & Geyer M. The RNA polymerase II carboxy-terminal domain (CTD) code. Chem. Rev. 113, 8456–8490 (2013). - PubMed
-
- Meinhart A. & Cramer P. Recognition of RNA polymerase II carboxy-terminal domain by 3′-RNA-processing factors. Nature 430, 223–226 (2004). - PubMed
-
- Cagas P. M. & Corden J. L. Structural studies of a synthetic peptide derived from the carboxyl-terminal domain of RNA polymerase II. Proteins 21, 149–160 (1995). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

