Direct Asymmetric Alkylation of Ketones: Still Unconquered
- PMID: 28497890
- PMCID: PMC6312110
- DOI: 10.1002/anie.201703079
Direct Asymmetric Alkylation of Ketones: Still Unconquered
Abstract
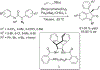
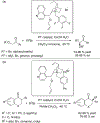
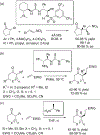
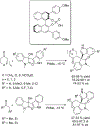
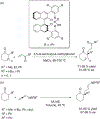
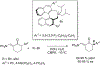
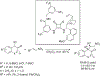
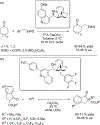
The alkylation of ketones is taught at basic undergraduate level. In many cases this transformation leads to the formation of a new stereogenic center. However, the apparent simplicity of the transformation is belied by a number of problems. So much so, that a general method for the direct asymmetric alkylation of ketones remains an unmet target. Despite the advancement of organocatalysis and transition-metal catalysis, neither field has provided an adequate solution. Indeed, even use of an efficient and general stoichiometric chiral reagent has yet to be reported. Herein we describe the state-of-the-art in terms of direct alkylation reactions of some carbonyl groups. We outline the limited progress that has been made with ketones, and potential routes towards ultimately achieving a widely applicable methodology for the asymmetric alkylation of ketones.
Keywords: asymmetric; ketones; organocatalysis; transition-metal catalysis; α-alkylation.
© 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
Conflict of interest
The authors declare no conflict of interest.
Figures
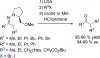
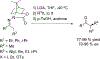
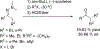

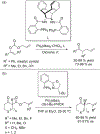

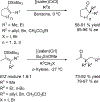
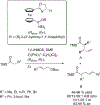
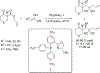
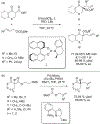
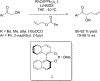
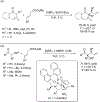
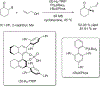
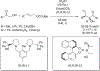
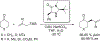
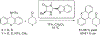
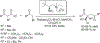
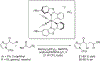
References
-
- Smith MB, March Jin March’s Advanced Organic Chemistry, Wiley, New York, 2001;
- Vesely J, Rios R, ChemCatChem 2012, 4, 942–953;
- Reeves CM, Stoltz BM in Asymmetric Synthesis: More Methods and Applications (Eds.: Christmann M, Bräse N), Wiley-VCH, Weinheim, 2012, pp. 1–10;
- Kohler MC, Wengryniuk SE, Coltart DM in Stereoselective Synthesis of Drugs and Natural Products (Eds.: Andrushko V, Andrushko N), Wiley, Hoboken, 2013, pp. 183–213;
- Remes M, Vesely J in Stereoselective Organocatalysis Bond Formation Methodologies and Activation Modes (Ed.: Torres R. Rios), Wiley, New York, 2013, pp. 267–312;
- Liu Y, Han S-J, Liu W-B, Stoltz BM, Acc. Chem. Res 2015,48,740–751; - PMC - PubMed
- Kazmaier U, Org. Chem. Front 2016, 3, 1541–1560.
-
- Ibrahem I, Cordova A, Angew. Chem. Int. Ed 2006, 45, 1952–1956; Angew. Chem. 2006, 118, 1986–1990; - PubMed
- Beeson TD, Mastracchio A, Hong J-B, Ashton K, MacMillan DWC, Science 2007, 316, 582–585; - PubMed
- Wilson JE, Casarez AD, MacMillan DWC, J. Am. Chem. Soc 2009, 131, 11332–11334; - PMC - PubMed
- Gomez-Bengoa E, Landa A, Lizarraga A, Mielgo A, Oiarbide M, Palomo C, Chem. Sci 2011, 2, 353–357;
- Arceo E, Jurberg ID, Álvarez-Fernández A, Melchiorre P, Nat. Chem 2013,5,750–756; - PubMed
- Bahamonde A, Melchiorre P, J. Am. Chem. Soc 2016,138, 8019–8030. - PMC - PubMed
-
- Ma Y, Stivala CE, Wright AM, Hayton T, Liang J, Keresztes I, Lobkovsky E, Collum DB, Zakarian A, J. Am. Chem. Soc 2013,135,16853–16864; - PMC - PubMed
- Lu P, Jackson JJ, Eickhoff JA, Zakarian A, J. Am. Chem. Soc 2015, 137, 656–659; - PMC - PubMed
- Yu K, Lu P, Jackson JJ, Nguyen T-AD, Alvarado J, Stivala CE, Ma Y, Mack KA, Hayton TW, Collum DB, Zakarian A, J. Am. Chem. Soc 2017,139, 527–533. - PMC - PubMed
-
- Evans DA in Asymmetric Synthesis, Vol. 3 (Ed.: Morrison JD), Academic Press, Orlando, 1984, pp. 1–10;
- Enders D in Asymmetric Synthesis, Vol. 3, 1st ed. (Ed.: Morrison JD), Academic Press, Orlando, 1984, pp. 275–339;
- Mekelburger HB, Wilcox CS in Comprehensive Organic Synthesis, Vol. 2 (Eds.: Trost BM, Fleming I), Pergamon, Oxford, 1991, pp. 99–131;
- Heatcock CH in Modern Synthetic Methods, Vol. 6 (Ed.: Scheffold R), Helvetica Chimica Acta, Basel, 1992, pp. 183–213;
- Rizzacasa MA, Perkins M in Stoichiometric Asymmetric Synthesis, Sheffield Academic Press, Sheffield, 2000;
- Carey FA, Sundberg RJ in Advanced Organic Chemistry Part B: Reactions and Synthesis, 5th ed., Springer, New York, 2007.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

