Usefulness of In Vivo and In Vitro Diagnostic Tests in the Diagnosis of Hypersensitivity Reactions to Quinolones and in the Evaluation of Cross-Reactivity: A Comprehensive Study Including the Latest Quinolone Gemifloxacin
- PMID: 28497922
- PMCID: PMC5446950
- DOI: 10.4168/aair.2017.9.4.347
Usefulness of In Vivo and In Vitro Diagnostic Tests in the Diagnosis of Hypersensitivity Reactions to Quinolones and in the Evaluation of Cross-Reactivity: A Comprehensive Study Including the Latest Quinolone Gemifloxacin
Abstract
Purpose: Reports evaluating diagnosis and cross reactivity of quinolone hypersensitivity have revealed contradictory results. Furthermore, there are no reports investigating the cross-reactivity between gemifloxacin (GFX) and the others. We aimed to detect the usefulness of diagnostic tests of hypersensitivity reactions to quinolones and to evaluate the cross reactivity between different quinolones including the latest quinolone GFX.
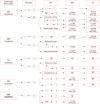
Methods: We studied 54 patients (mean age 42.31±10.39 years; 47 female) with 57 hypersensitivity reactions due to different quinolones and 10 nonatopic quinolone tolerable control subjects. A detailed clinical history, skin test (ST), and single-blind placebo-controlled drug provocation test (SBPCDPT), as well as basophil activation test (BAT) and lymphocyte transformation test (LTT) were performed with the culprit and alternative quinolones including ciprofloxacin (CFX), moxifloxacin (MFX), levofloxacin (LFX), ofloxacin (OFX), and GFX.
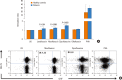
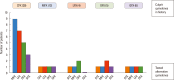
Results: The majority (75.9%) of the patients reported immediate type reactions to various quinolones. The most common culprit drug was CFX (52.6%) and the most common reaction type was urticaria (26.3%). A quarter of the patients (24.1%) reacted to SBPCDPTs, although their STs were negative; while false ST positivity was 3.5% and ST/SBPCDPTs concordance was only 1.8%. Both BAT and LTT were not found useful in quinolone hypersensitivity. Cross-reactivity was primarily observed between LFX and OFX (50.0%), whereas it was the least between MFX and the others, and in GFX hypersensitive patients the degree of cross-reactivity to the other quinolones was 16.7%.
Conclusions: These results suggest that STs, BAT, and LTT are not supportive in the diagnosis of a hypersensitivity reaction to quinolone as well as in the prediction of cross-reactivity. Drug provocation tests (DPTs) are necessary to identify both culprit and alternative quinolones.
Keywords: Quinolone hypersensitivity; basophil activation test; lymphocyte transformation test.
Copyright © 2017 The Korean Academy of Asthma, Allergy and Clinical Immunology · The Korean Academy of Pediatric Allergy and Respiratory Disease
Conflict of interest statement
There are no financial or other issues that might lead to conflict of interest.
Figures

Similar articles
-
Update on Quinolone Allergy.Curr Allergy Asthma Rep. 2017 Aug;17(8):56. doi: 10.1007/s11882-017-0725-y. Curr Allergy Asthma Rep. 2017. PMID: 28752367 Review.
-
Allergy to quinolones: low cross-reactivity to levofloxacin.J Investig Allergol Clin Immunol. 2010;20(7):607-11. J Investig Allergol Clin Immunol. 2010. PMID: 21314003
-
Clinical Characterization and Diagnostic Approaches for Patients Reporting Hypersensitivity Reactions to Quinolones.J Allergy Clin Immunol Pract. 2020 Sep;8(8):2707-2714.e2. doi: 10.1016/j.jaip.2020.04.051. Epub 2020 May 4. J Allergy Clin Immunol Pract. 2020. PMID: 32376487
-
Diagnostic workup including CD203c-based basophil activation test in immediate hypersensitivity due to metronidazole and ornidazole and evaluation of cross-reactivity in between.Allergy. 2021 Mar;76(3):842-852. doi: 10.1111/all.14542. Epub 2020 Aug 27. Allergy. 2021. PMID: 32761620 Clinical Trial.
-
Quinolone Allergy.Pharmacy (Basel). 2019 Jul 19;7(3):97. doi: 10.3390/pharmacy7030097. Pharmacy (Basel). 2019. PMID: 31330937 Free PMC article. Review.
Cited by
-
An unusual dual hypersensitivity reaction to moxifloxacin in a patient.Asia Pac Allergy. 2018 Jul 17;8(3):e26. doi: 10.5415/apallergy.2018.8.e26. eCollection 2018 Jul. Asia Pac Allergy. 2018. PMID: 30079304 Free PMC article.
-
Update on Quinolone Allergy: A Complementary Note.Curr Allergy Asthma Rep. 2017 Oct 3;17(10):74. doi: 10.1007/s11882-017-0742-x. Curr Allergy Asthma Rep. 2017. PMID: 28975524 No abstract available.
-
Update on Quinolone Allergy.Curr Allergy Asthma Rep. 2017 Aug;17(8):56. doi: 10.1007/s11882-017-0725-y. Curr Allergy Asthma Rep. 2017. PMID: 28752367 Review.
-
Is a Drug Allergy in a Patient's History Real? Our Experience with Diagnostic Drug Provocation Tests.Medicina (Kaunas). 2025 Feb 23;61(3):386. doi: 10.3390/medicina61030386. Medicina (Kaunas). 2025. PMID: 40142197 Free PMC article.
-
In-Class Cross-Reactivity among Hospitalized Patients with Hypersensitivity Reactions to Fluoroquinolones.Antimicrob Agents Chemother. 2023 Jun 15;67(6):e0037423. doi: 10.1128/aac.00374-23. Epub 2023 May 8. Antimicrob Agents Chemother. 2023. PMID: 37154772 Free PMC article.
References
-
- Van Bambeke F, Michot JM, Van Eldere J, Tulkens PM. Quinolones in 2005: an update. Clin Microbiol Infect. 2005;11:256–280. - PubMed
-
- Ball P. Quinolone generations: natural history or natural selection? J Antimicrob Chemother. 2000;46:17–24. - PubMed
-
- Bertino J, Jr, Fish D. The safety profile of the fluoroquinolones. Clin Ther. 2000;22:798–817. - PubMed
-
- Doña I, Blanca-López N, Torres MJ, García-Campos J, García-Núñez I, Gómez F, et al. Drug hypersensitivity reactions: response patterns, drug involved, and temporal variations in a large series of patients. J Investig Allergol Clin Immunol. 2012;22:363–371. - PubMed
-
- Sachs B, Riegel S, Seebeck J, Beier R, Schichler D, Barger A, et al. Fluoroquinolone-associated anaphylaxis in spontaneous adverse drug reaction reports in Germany: differences in reporting rates between individual fluoroquinolones and occurrence after first-ever use. Drug Saf. 2006;29:1087–1100. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

