Autism Behavior Inventory: A Novel Tool for Assessing Core and Associated Symptoms of Autism Spectrum Disorder
- PMID: 28498053
- PMCID: PMC5689117
- DOI: 10.1089/cap.2017.0018
Autism Behavior Inventory: A Novel Tool for Assessing Core and Associated Symptoms of Autism Spectrum Disorder
Abstract
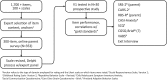
Objective: Autism Behavior Inventory (ABI) is a new measure for assessing changes in core and associated symptoms of autism spectrum disorder (ASD) in participants (ages: 3 years-adulthood) diagnosed with ASD. It is a web-based tool with five domains (two ASD core domains: social communication, restrictive and repetitive behaviors; three associated domains: mental health, self-regulation, and challenging behavior). This study describes design, development, and initial psychometric properties of the ABI.
Methods: ABI items were generated following review of existing measures and inputs from expert clinicians. Initial ABI scale contained 161 items that were reduced to fit a factor analytic model, retaining items of adequate reliability. Two versions of the scale, ABI-full (ABI-F; 93 items) and ABI-short version (ABI-S; 36 items), were developed and evaluated for psychometric properties, including validity comparisons with commonly used measures. Both scales were administered to parents and healthcare professionals (HCPs) involved with study participants.
Results: Test-retest reliability (intraclass correlation coefficient [ICC] = 0.79) for parent ratings on ABI was robust and compared favorably to existing scales. Test-retest correlations for HCP ratings were generally lower versus parent ratings. ABI core domains and comparison measures strongly correlated (r ≥ 0.70), demonstrating good concurrent validity.
Conclusions: Overall, ABI demonstrates promise as a tool for measuring change in core symptoms of autism in ASD clinical studies, with further validation required.
Keywords: assessment; autism spectrum disorder; measures; outcome; rating scale; software.
References
-
- Achenbach TM, McConaughy SH, Howell CT: Child/adolescent behavioral and emotional problems: Implications of cross-informant correlations for situational specificity. Psychol Bull 101:213–232, 1987 - PubMed
-
- Achenbach TM, Rescorla LA: Manual for the ASEBA School-Age Forms & Profiles. Burlington, Research Centre for Children, Youth and Families, University of Vermont, 2001
-
- Akhondzadeh S, Tajdar H, Mohammadi MR: A double-blind placebo controlled trial of piracetam added to risperidone in patients with autistic disorder. Child Psychiatry Hum Dev 39:237–245, 2008 - PubMed
-
- Aman MG, Arnold LE. and Hollway JA: Assessing change in core autism symptoms: Challenges for pharmacological studies. J Child Adolesc Psychopharmacol 25:282–285, 2015 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous