Intracellular uptake of macromolecules by brain lymphatic endothelial cells during zebrafish embryonic development
- PMID: 28498105
- PMCID: PMC5457137
- DOI: 10.7554/eLife.25932
Intracellular uptake of macromolecules by brain lymphatic endothelial cells during zebrafish embryonic development
Abstract
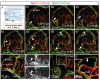
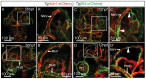
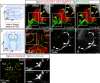
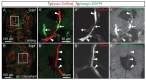
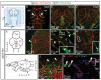
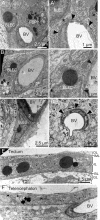
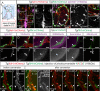
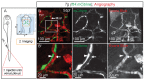
The lymphatic system controls fluid homeostasis and the clearance of macromolecules from interstitial compartments. In mammals brain lymphatics were only recently discovered, with significant implications for physiology and disease. We examined zebrafish for the presence of brain lymphatics and found loosely connected endothelial cells with lymphatic molecular signature covering parts of the brain without forming endothelial tubular structures. These brain lymphatic endothelial cells (BLECs) derive from venous endothelium, are distinct from macrophages, and are sensitive to loss of Vegfc. BLECs endocytose macromolecules in a selective manner, which can be blocked by injection of mannose receptor ligands. This first report on brain lymphatic endothelial cells in a vertebrate embryo identifies cells with unique features, including the uptake of macromolecules at a single cell level. Future studies will address whether this represents an uptake mechanism that is conserved in mammals and how these cells affect functions of the embryonic and adult brain.
Keywords: brain; developmental biology; endothelial cell; lymphatics; stem cells; zebrafish.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures


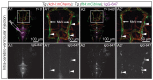

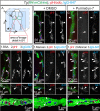

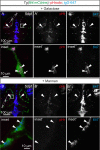
References
-
- Aspelund A, Tammela T, Antila S, Nurmi H, Leppänen VM, Zarkada G, Stanczuk L, Francois M, Mäkinen T, Saharinen P, Immonen I, Alitalo K. The Schlemm's canal is a VEGF-C/VEGFR-3-responsive lymphatic-like vessel. Journal of Clinical Investigation. 2014;124:3975–3986. doi: 10.1172/JCI75395. - DOI - PMC - PubMed
-
- Bos FL, Caunt M, Peterson-Maduro J, Planas-Paz L, Kowalski J, Karpanen T, van Impel A, Tong R, Ernst JA, Korving J, van Es JH, Lammert E, Duckers HJ, Schulte-Merker S. CCBE1 is essential for mammalian lymphatic vascular development and enhances the lymphangiogenic effect of vascular endothelial growth factor-C in vivo. Circulation Research. 2011;109:486–491. doi: 10.1161/CIRCRESAHA.111.250738. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

