Viral Suppression and HIV Drug Resistance at 6 Months Among Women in Malawi's Option B+ Program: Results From the PURE Malawi Study
- PMID: 28498184
- PMCID: PMC5431274
- DOI: 10.1097/QAI.0000000000001368
Viral Suppression and HIV Drug Resistance at 6 Months Among Women in Malawi's Option B+ Program: Results From the PURE Malawi Study
Abstract
Background: In 2011, Malawi launched Option B+, a program of universal antiretroviral therapy (ART) treatment for pregnant and lactating women to optimize maternal health and prevent pediatric HIV infection. For optimal outcomes, women need to achieve HIVRNA suppression. We report 6-month HIVRNA suppression and HIV drug resistance in the PURE study.
Methods: PURE study was a cluster-randomized controlled trial evaluating 3 strategies for promoting uptake and retention; arm 1: Standard of Care, arm 2: Facility Peer Support, and arm 3: Community Peer support. Pregnant and breastfeeding mothers were enrolled and followed according to Malawi ART guidelines. Dried blood spots for HIVRNA testing were collected at 6 months. Samples with ART failure (HIVRNA ≥1000 copies/ml) had resistance testing. We calculated odds ratios for ART failure using generalized estimating equations with a logit link and binomial distribution.
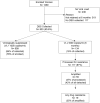
Results: We enrolled 1269 women across 21 sites in Southern and Central Malawi. Most enrolled while pregnant (86%) and were WHO stage 1 (95%). At 6 months, 950/1269 (75%) were retained; 833/950 (88%) had HIVRNA testing conducted, and 699/833 (84%) were suppressed. Among those with HIVRNA ≥1000 copies/ml with successful amplification (N = 55, 41% of all viral loads > 1000 copies/ml), confirmed HIV resistance was found in 35% (19/55), primarily to the nonnucleoside reverse transcriptase inhibitor class of drugs. ART failure was associated with treatment default but not study arm, age, WHO stage, or breastfeeding status.
Conclusions: Virologic suppression at 6 months was <90% target, but the observed confirmed resistance rates suggest that adherence support should be the primary approach for early failure in option B+.
Trial registration: ClinicalTrials.gov NCT02005835.
Conflict of interest statement
Figures
References
-
- Schouten EJ, Jahn A, Midiani D, et al. Prevention of mother-to-child transmission of HIV and the health-related Millennium Development Goals: time for a public health approach. Lancet. 2011 Jul 16;378(9787):282–284. - PubMed
-
- Ministry of Health Malawi. Clinical Management of HIV in Adults and Children: Malawi Integrated Guidelines for Providing Service in: Antenatal Care, Maternity Care, Under Five Clinics, Family Planning Clinics, Exposed Infant/Pre-ART clinics, ART clinics. 1st. Lilongwe: Ministry of Health, Malawi; 2011. [accessed 9, 2017]. https://www.hiv.health.gov.mw.
-
- Health MMo, editor. Department of HIV and AIDS Ministry of Health. Malawi Integrated HIV Program Report for Oct-Dec 2013. Lilongwe: 2013.
-
- Speight C, Phiri SPS, Hosseinipour M, Tweya H, Chimbwandira F, Chikonda J, Phoso M, Kalulu M, Chauma A. IAS 2013: 7th IAS conference on HIV pathogenesis, Treatment, and Prevention. Kuala Lumpur, Malaysia: 2013. [accessed 9, 2017]. Implementing Option B+ for prevention of mother to child transmission at Bwaila Maternity Unit, Lilongwe: the first 18 months. http://pag.ias2013.org/abstracts. Abstract Number WELBCO1.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical