Initiation and execution mechanisms of necroptosis: an overview
- PMID: 28498367
- PMCID: PMC5520172
- DOI: 10.1038/cdd.2017.65
Initiation and execution mechanisms of necroptosis: an overview
Abstract
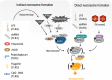
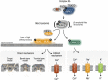
Necroptosis is a form of regulated cell death, which is induced by ligand binding to TNF family death domain receptors, pattern recognizing receptors and virus sensors. The common feature of these receptor systems is the implication of proteins, which contain a receptor interaction protein kinase (RIPK) homology interaction motif (RHIM) mediating recruitment and activation of receptor-interacting protein kinase 3 (RIPK3), which ultimately activates the necroptosis executioner mixed lineage kinase domain-like (MLKL). In case of the TNF family members, the initiator is the survival- and cell death-regulating RIPK1 kinase, in the case of Toll-like receptor 3/4 (TLR3/4), a RHIM-containing adaptor, called TRIF, while in the case of Z-DNA-binding protein ZBP1/DAI, the cytosolic viral sensor itself contains a RHIM domain. In this review, we discuss the different protein complexes that serve as nucleation platforms for necroptosis and the mechanism of execution of necroptosis. Transgenic models (knockout, kinase-dead knock-in) and pharmacologic inhibition indicate that RIPK1, RIPK3 or MLKL are implicated in many inflammatory, degenerative and infectious diseases. However, the conclusion of necroptosis being solely involved in the etiology of diseases is blurred by the pleiotropic roles of RIPK1 and RIPK3 in other cellular processes such as apoptosis and inflammasome activation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

