Mitochondrial activity and oxidative stress functions are influenced by the activation of AhR-induced CYP1A1 overexpression in cardiomyocytes
- PMID: 28498411
- PMCID: PMC5482149
- DOI: 10.3892/mmr.2017.6580
Mitochondrial activity and oxidative stress functions are influenced by the activation of AhR-induced CYP1A1 overexpression in cardiomyocytes
Abstract
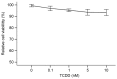
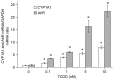
There is an endemic cardiomyopathy currently occurring in China, termed, Keshan disease (KD). The authors previously compared mitochondrial‑associated gene expression profiles of peripheral blood mononuclear cells (PBMCs) derived from KD patients and normal controls, using mitochondria‑focused cDNA microarray technology. The results detected an upregulation of the enzyme‑associated CYP1A1 gene, (ratios ≥2.0). The aryl hydrocarbon receptor (AhR) regulates the expression of numerous cytochrome P450 (CYP) genes including members of the CYP1 family; CYP1A1 and CYP1A2. Several previous studies have suggested roles for the aryl hydrocarbon receptor (AhR) and the genes that it regulates. An example involves cytochrome P4501A1 (CYP1A1), in the pathogenesis of heart failure, cardiac hypertrophy and other cardiomyopathies. Mitochondria comprise ~30% of the intracellular volume in mammalian cardiomyocytes, and subtle alterations in mitochondria can markedly influence cardiomyopathies. The present study investigated alterations in the activity and functions of mitochondria following AhR‑induced overexpression of CYP1A1. AC16 cells were treated with the CYP1A1 inducer 2,3,7,8‑tetrachlorodibenzo‑p‑dioxin (TCDD), and cytotoxicity was then evaluated in MTT assays. Reverse transcription‑quantitative polymerase chain reactions, western blot analysis and 7‑ethoxyresorufin O‑deacylase assays were performed to analyze the mRNA and protein levels, and the enzymatic activity of CYP1A1. Mitochondrial activity and mass were analyzed using an inverted fluorescence microscope and a fluorescence microplate reader. Reactive oxygen species (ROS) activity was analyzed using flow cytometry. The results of the current study demonstrated that TCDD gradually increased mRNA and protein levels of AhR and CYP1A1, in addition to the enzymatic activity. Mitochondrial activity and the quality of mitochondrial membranes were also significantly attenuated, and mitochondrial ROS levels were elevated in the TCDD‑induced cardiomyocytes. The results indicate the involvement of the AhR/CYP1A1 signaling pathway in the mechanism of action of TCDD in human cardiomyocytes. The present findings may provide an explanation for myocardial injuries caused by polycyclic aromatic hydrocarbons. The authors conclude that exposure to TCDD results in regulatory alteration to the expression of detoxification genes that ultimately affect the metabolic activation and function of cardiomyocytes.
Figures





References
-
- Kann S, Huang MY, Estes C, Reichard JF, Sartor MA, Xia Y, Puga A. Arsenite-induced aryl hydrocarbon receptor nuclear translocation results in additive induction of phase I genes and synergistic induction of phase II genes. Mol Pharmacol. 2005;68:336–346. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

