Varenicline versus nicotine patch with brief advice for smokers with substance use disorders with or without depression: effects on smoking, substance use and depressive symptoms
- PMID: 28498504
- PMCID: PMC12435192
- DOI: 10.1111/add.13861
Varenicline versus nicotine patch with brief advice for smokers with substance use disorders with or without depression: effects on smoking, substance use and depressive symptoms
Abstract
Aims: Varenicline was compared with transdermal nicotine (NRT) for smokers with current substance use disorders (SUD) for effects on 3-month smoking abstinence (primary outcome) and, secondarily, on 3- and 6 month abstinence while adjusting for medication adherence, and on additional smoking and substance use outcomes. Moderation by major depressive disorder history (MDD) and adherence were investigated.
Design: Double-blind double-placebo-controlled randomized design, stratifying by MDD, gender and nicotine dependence, with 3 and 6 months follow-up.
Setting: University offices in Rhode Island, USA.
Participants: Adult smokers (n = 137), in SUD treatment, substance abstinent <12 months (n = 77 varenicline, 60 NRT).
Intervention and comparator: Twelve weeks of varenicline (2 mg/day, after 1-week dose run-up) or NRT (21 mg/day decreasing to 7 mg/day).
Measurements: Primary: point-prevalence smoking abstinence (7-day, confirmed) at 3 months. Secondary: point-prevalence abstinence at 6 months, quantity and frequency of smoking and substance use at 3 and 6 months, and within-treatment abstinence, medication adherence and depressive symptoms. Smoking outcome analyses were repeated controlling for adherence and investigating adherence as a moderator.
Findings: Effects on 3-month abstinence were P < 0.065 without a covariate (Bayes factor 3.35, supporting the effect strongly) and differed significantly when controlling for baseline smoking [varenicline: 13%, NRT: 3%; odds ratio (OR) = 4.81, 95% confidence interval (CI) 1.00, 23.13, P < 0.05]. The threefold difference at 6 months was not significant. Medication effect on abstinence across time was significant (P < 0.05) covarying adherence and baseline smoking (OR = 6.40, 95% CI = 1.00, 40.93). Medication differences in 3-month abstinence occurred among participants with ≥ 77% adherence (P < 0.02). No significant medication effects on heavy drinking, drug use or depressive symptoms were found.
Conclusions: Varenicline appears to improve the chances of achieving at least 3 months of smoking abstinence in smokers with substance use disorders trying to stop, compared with transdermal nicotine patches, the effect being independent of history of depressive disorder.
Keywords: Brief advice; depression; nicotine dependence; point-prevalence abstinence; smoking cessation; substance use disorders; varenicline.
© 2017 Society for the Study of Addiction.
Conflict of interest statement
Declaration of interests
All authors report no financial interests or potential conflicts of interest except for the following: R.M.S. has received travel and honorarium from D&A Pharma and has received consultant fees from CT Laboratories.
Figures
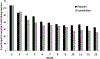

References
-
- Hurt RD, Patten CA Treatment of tobacco dependence in alcoholics. Recent Dev Alcohol 2003; 16: 335–59. - PubMed
-
- Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, Morse RM et al. Mortality following inpatient addictions treatment: Role of tobacco use in a community-based cohort. JAMA 1996; 275: 1097–103. - PubMed
-
- Zacny JP Behavioral aspects of alcohol-tobacco interactions. In: Galanter M, editor. Recent Developments in Alcoholism: Volume 8. Combined Alcohol and Other Drug Dependence. New York: Plenum Press; 1990, pp. 205–19. - PubMed
-
- Hughes JR Treatment of smoking cessation in smokers with past alcohol/drug problems. J Subst Abuse Treat 1993; 10: 181–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

