Has the rising placebo response impacted antidepressant clinical trial outcome? Data from the US Food and Drug Administration 1987-2013
- PMID: 28498591
- PMCID: PMC5428172
- DOI: 10.1002/wps.20421
Has the rising placebo response impacted antidepressant clinical trial outcome? Data from the US Food and Drug Administration 1987-2013
Erratum in
-
Correction.World Psychiatry. 2017 Oct;16(3):328. doi: 10.1002/wps.20469. World Psychiatry. 2017. PMID: 28941120 Free PMC article. No abstract available.
Abstract
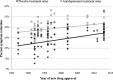
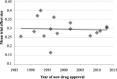
More than fifteen years ago, it was noted that the failure rate of antidepressant clinical trials was high, and such negative outcomes were thought to be related to the increasing magnitude of placebo response. However, there is considerable debate regarding this phenomenon and its relationship to outcomes in more recent antidepressant clinical trials. To investigate this, we accessed the US Food and Drug Administration (FDA) reviews for sixteen antidepressants (85 trials, 115 trial arms, 23,109 patients) approved between 1987 and 2013. We calculated the magnitude of placebo and antidepressant responses, antidepressant-placebo differences, as well as the effect sizes and success rates, and compared these measures over time. Exploratory analysis investigated potential changes in trial design and conduct over time. As expected, the magnitude of placebo response has steadily grown in the past 30 years, increasing since 2000 by 6.4% (r=0.46, p<0.001). Contrary to expectations, a similar increase has occurred in the magnitude of antidepressant response (6.0%, r=0.37, p<0.001). Thus, the effect sizes (0.30 vs. 0.29, p=0.42) and the magnitude of antidepressant-placebo differences (10.5% vs. 10.3%, p=0.37) have remained statistically equivalent. Furthermore, the frequency of positive trial arms has gone up in the past 15 years (from 47.8% to 63.8%), but this difference in frequency has not reached statistical significance. Trial design features that were previously associated with a possible lower magnitude of placebo response were not implemented, and their relationship to the magnitude of placebo response could not be replicated. Of the 34 recent trials, two implemented enhanced interview techniques, but both of them were unsuccessful. The results of this study suggest that the relationship between the magnitude of placebo response and the outcome of antidepressant clinical trials is weak at best. These data further indicate that antidepressant-placebo differences are about the same for all of the sixteen antidepressants approved by the FDA in the past thirty years.
Keywords: Antidepressants; antidepressant-placebo difference; clinical trials; effect size; enhanced interview techniques; placebo response; success rate.
© 2017 World Psychiatric Association.
Figures
Comment in
-
Is placebo response in antidepressant trials rising or not? A reanalysis of datasets to conclude this long-lasting controversy.Evid Based Ment Health. 2018 Feb;21(1):1-3. doi: 10.1136/eb-2017-102827. Epub 2018 Jan 12. Evid Based Ment Health. 2018. PMID: 29330216 Free PMC article.
References
-
- Khan A, Warner HA, Brown WA. Symptom reduction and suicide risk in patients treated with placebo in antidepressant clinical trials. Arch Gen Psychiatry 2000;57:311‐7. - PubMed
-
- Khan A, Khan S, Brown WA. Are placebo controls necessary to test new antidepressants and anxiolytics? Int J Neuropsychopharmacol 2002;5:193‐7. - PubMed
-
- Walsh BT, Seidman SN, Sysko R et al. Placebo response in studies of major depression: variable, substantial, and growing. JAMA 2001;287:1840‐7. - PubMed
-
- Khan A, Detke M, Khan S et al. Placebo response and antidepressant clinical trial outcome. J Nerv Ment Dis 2003;191:211‐8. - PubMed
-
- Rief W, Nestoriuc Y, Weiss S et al. Meta‐analysis of the placebo response in antidepressant trials. J Affect Disord 2009;118:1‐8. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

