A family affair: A Ral-exocyst-centered network links Ras, Rac, Rho signaling to control cell migration
- PMID: 28498728
- PMCID: PMC6748358
- DOI: 10.1080/21541248.2017.1310649
A family affair: A Ral-exocyst-centered network links Ras, Rac, Rho signaling to control cell migration
Abstract
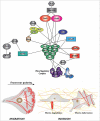
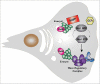
Cell migration is central to many developmental, physiologic and pathological processes, including cancer progression. The Ral GTPases (RalA and RalB) which act down-stream the Ras oncogenes, are key players in the coordination between membrane trafficking and actin polymerization. A major direct effector of Ral, the exocyst complex, works in polarized exocytosis and is at the center of multiple protein-protein interactions that support cell migration by promoting protrusion formation, front-rear polarization, and extra-cellular matrix degradation. In this review we describe the recent advancements in deciphering the molecular mechanisms underlying this role of Ral via exocyst on cell migration. Among others, we will discuss the recently identified cross-talk between Ral and Rac1 pathways: exocyst binds to a negative regulator (the RacGAP SH3BP1) and to the major effector (the Wave Regulatory Complex, WRC) of Rac1, the master regulator of protrusions. Next challenge will be to better characterize the dynamics in space and in time of these molecular interplays, to better understand the pleiotropic functions of Ral in both normal and cancer cells.
Keywords: Rac; Ral; Ras; Rho; Wave; exocyst; migration.
Figures
References
-
- Camonis JH, White MA. Ral GTPases: corrupting the exocyst in cancer cells. Trends Cell Biol 2005; 15:327-32; PMID:15953551; https://doi.org/10.1016/j.tcb.2005.04.002 - DOI - PubMed
-
- Shirakawa R, Horiuchi H. Ral GTPases: crucial mediators of exocytosis and tumourigenesis. J Biochem (Tokyo) 2015; 157:285-99; https://doi.org/10.1093/jb/mvv029 - DOI - PubMed
-
- Gentry LR, Martin TD, Reiner DJ, Der CJ. Ral small GTPase signaling and oncogenesis: More than just 15 minutes of fame. Biochim Biophys Acta 2014; 1843:2976-88; PMID:25219551; https://doi.org/10.1016/j.bbamcr.2014.09.004 - DOI - PMC - PubMed
-
- Lee T, Feig L, Montell DJ. Two distinct roles for Ras in a developmentally regulated cell migration. Dev Camb Engl 1996; 122:409-18 - PubMed
-
- Takaya A, Ohba Y, Kurokawa K, Matsuda M. RalA activation at nascent lamellipodia of epidermal growth factor-stimulated Cos7 cells and migrating Madin-Darby canine kidney cells. Mol Biol Cell 2004; 15:2549-57; PMID:15034142; https://doi.org/10.1091/mbc.E03-11-0857 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
