Tissue transglutaminase expression is necessary for adhesion, metastatic potential and cancer stemness of renal cell carcinoma
- PMID: 28498731
- PMCID: PMC5927635
- DOI: 10.1080/19336918.2017.1322255
Tissue transglutaminase expression is necessary for adhesion, metastatic potential and cancer stemness of renal cell carcinoma
Abstract
Tissue transglutaminase (TG2) is the ubiquitously expressed member of transglutaminase family and shown to play a critical role in the development and progression of drug resistance malignancies. We have previously showed the association of TG2 upregulation with progression and metastasis of renal cell carcinoma (RCC) and low disease-free survival. In the present study we further investigate the role of TG2 in cell adhesion, migration and invasion of RCC by silencing TG2 expression in Caki-2 and A-498 primary site and Caki-1 and ACHN metastatic site RCC cell lines. Downregulation of TG2 expression led up to a 60% decrease in actin stress fiber formation and adhesion to β 1 integrin (ITGB1) substrates fibronectin, collagen type I and laminin in both primary and metastatic site RCC cell lines. In addition, treatment with siRNAs against TG2 impaired the migration capacity and cellular invasiveness of ITGB1 substrates in all 4 RCC cell lines. Lastly, the knockdown of TG2 in metastatic Caki-1 cells diminished the expression of CD44, CD73-and CD105 cancer stem cell-like markers. We conclude, for the first time, that TG2 expression is critical for cancer cell adhesion, migration, invasiveness and cancer cell-stemness during RCC progression and dissemination. Therefore, combined targeting of TG2 with drugs widely used in the treatment of RCC may be a promising therapeutic strategy for RCC.
Keywords: cancer cell stemness; cell adhesion; invasion; renal cell carcinoma; tissue transglutaminase.
Figures
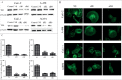
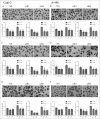

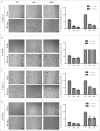
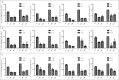
Similar articles
-
Comparative Gene Expression Profiling of Primary and Metastatic Renal Cell Carcinoma Stem Cell-Like Cancer Cells.PLoS One. 2016 Nov 3;11(11):e0165718. doi: 10.1371/journal.pone.0165718. eCollection 2016. PLoS One. 2016. PMID: 27812180 Free PMC article.
-
Up-regulation of fibronectin and tissue transglutaminase promotes cell invasion involving increased association with integrin and MMP expression in A431 cells.Anticancer Res. 2010 Oct;30(10):4177-86. Anticancer Res. 2010. PMID: 21036738
-
Up-regulation of TGM2 with ITGB1 and SDC4 is important in the development and metastasis of renal cell carcinoma.Urol Oncol. 2014 Jan;32(1):25.e13-20. doi: 10.1016/j.urolonc.2012.08.022. Epub 2013 Mar 15. Urol Oncol. 2014. PMID: 23499501
-
Transglutaminase is a tumor cell and cancer stem cell survival factor.Mol Carcinog. 2015 Oct;54(10):947-58. doi: 10.1002/mc.22375. Epub 2015 Aug 10. Mol Carcinog. 2015. PMID: 26258961 Free PMC article. Review.
-
Transglutaminase 2: The Maestro of the Oncogenic Mediators in Renal Cell Carcinoma.Med Sci (Basel). 2019 Feb 6;7(2):24. doi: 10.3390/medsci7020024. Med Sci (Basel). 2019. PMID: 30736384 Free PMC article. Review.
Cited by
-
The Outside-In Journey of Tissue Transglutaminase in Cancer.Cells. 2022 May 29;11(11):1779. doi: 10.3390/cells11111779. Cells. 2022. PMID: 35681474 Free PMC article. Review.
-
The Biological and Biomechanical Role of Transglutaminase-2 in the Tumour Microenvironment.Cancers (Basel). 2021 Jun 3;13(11):2788. doi: 10.3390/cancers13112788. Cancers (Basel). 2021. PMID: 34205140 Free PMC article. Review.
-
Identification of a novel cancer stem cell subpopulation that promotes progression of human fatal renal cell carcinoma by single-cell RNA-seq analysis.Int J Biol Sci. 2020 Oct 17;16(16):3149-3162. doi: 10.7150/ijbs.46645. eCollection 2020. Int J Biol Sci. 2020. PMID: 33162821 Free PMC article.
-
Fibronectin Promotes Cell Growth and Migration in Human Renal Cell Carcinoma Cells.Int J Mol Sci. 2019 Jun 7;20(11):2792. doi: 10.3390/ijms20112792. Int J Mol Sci. 2019. PMID: 31181623 Free PMC article.
-
Transglutaminase 2 takes center stage as a cancer cell survival factor and therapy target.Mol Carcinog. 2019 Jun;58(6):837-853. doi: 10.1002/mc.22986. Epub 2019 Mar 28. Mol Carcinog. 2019. PMID: 30693974 Free PMC article.
References
-
- Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell 2011; 147:275-92; PMID:22000009; https://doi.org/10.1016/j.cell.2011.09.024 - DOI - PMC - PubMed
-
- Seguin L, Desgrosellier JS, Weis SM, Cheresh DA. Integrins and cancer: regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol 2015; 25:234-40; PMID:25572304; https://doi.org/10.1016/j.tcb.2014.12.006 - DOI - PMC - PubMed
-
- Iwamoto DV, Calderwood DA. Regulation of integrin-mediated adhesions. Curr Opin Cell Biol 2015; 36:41-7; PMID:26189062; https://doi.org/10.1016/j.ceb.2015.06.009 - DOI - PMC - PubMed
-
- Ley K, Rivera-Nieves J, Sandborn WJ, Shattil S. Integrin-based therapeutics: biological basis, clinical use and new drugs. Nat Rev Drug Discov 2016; 15:173-83; PMID:26822833; https://doi.org/10.1038/nrd.2015.10 - DOI - PMC - PubMed
-
- Verderio EA, Telci D, Okoye A, Melino G, Griffin M. A novel RGD-independent cel adhesion pathway mediated by fibronectin-bound tissue transglutaminase rescues cells from anoikis. J Biol Chem 2003; 278:42604-14; PMID:12732629; https://doi.org/10.1074/jbc.M303303200 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
