Dietary luteolin attenuates chronic liver injury induced by mercuric chloride via the Nrf2/NF-κB/P53 signaling pathway in rats
- PMID: 28498799
- PMCID: PMC5522226
- DOI: 10.18632/oncotarget.17334
Dietary luteolin attenuates chronic liver injury induced by mercuric chloride via the Nrf2/NF-κB/P53 signaling pathway in rats
Abstract
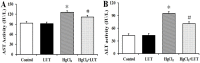
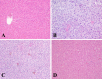
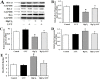
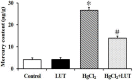
Mercury exposure is a common cause of metal poisoning which is biotransformed to highly toxic metabolites thus eliciting biochemical alterations and oxidative stress. Luteolin, a phenolic compound found in many natural products, has multiple biological functions. Our study was aimed to explore the biological effects of luteolin in a liver injury model induced in rats by mercuric chloride (HgCl2). Criteria for injury included liver enzyme, glutathione and malondialdehyde levels, histopathology, TUNEL assay, hepatocyte viability and reactive oxygen species levels. The results showed that luteolin protected against HgCl2-induced liver injury. Luteolin increased total nuclear factor-erythroid-2-related factor 2 (Nrf2) levels in the presence of HgCl2. Upregulation of its downstream factors, heme oxygenase-1 and NAD(P)H quinone oxidoreductase 1, was also observed. This suggested that protection by luteolin against HgCl2-induced liver injury involved Nrf2 pathway activation. Luteolin also decreased expression of nuclear factor-κB (NF-κB) and P53. HgCl2 exposure led to increased Bcl-associated X protein (Bax), and decreased Bcl-2-related protein long form of Bcl-x (Bcl-xL) and B-cell leukemia/lymphoma-2 (Bcl-2) expression, leading to an increased Bax/Bcl-2 ratio. Taken together, our data suggested that decreasing oxidative stress is a protective mechanism of luteolin against development of HgCl2-induced liver injury, through the Nrf2/NF-κB/P53 signaling pathway in rats.
Keywords: HgCl2; NF-κB; Nrf2; hepatotoxicity; luteolin.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures


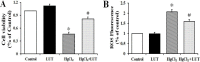




References
-
- Emsley J. Oxford University Press; 2001. Nature's building blocks: An A–Z guide to the elements.
-
- Perottoni J, Lobato LP, Silveira A, Rocha JB, Emanuelli T. Effects of mercury and selenite on delta-aminolevulinate dehydratase activity and on selected oxidative stress parameters in rats. Environ Res. 2004;95:166–73. - PubMed
-
- Moraes-Silva L, Bueno TM, Franciscato C, Oliveira CS, Peixoto NC, Pereira ME. Mercury chloride increases hepatic alanine aminotransferase and glucose 6-phosphatase activities in newborn rats in vivo. Cell Biol Int. 2012;36:561–6. - PubMed
-
- Zhang J, Lu S, Wang H, Zheng Q. Protective role of Aralia elata polysaccharide on mercury(II)-induced cardiovascular oxidative injury in rats. Int J Biol Macromol. 2013;59:301–4. - PubMed
-
- McFee RB, Caraccio TR. Intravenous mercury injection and ingestion: clinical manifestations and management. J Toxicol Clin Toxicol. 2001;39:733–8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

