The generation and functional characterization of induced pluripotent stem cells from human intervertebral disc nucleus pulposus cells
- PMID: 28498811
- PMCID: PMC5522099
- DOI: 10.18632/oncotarget.17446
The generation and functional characterization of induced pluripotent stem cells from human intervertebral disc nucleus pulposus cells
Abstract
Disc degenerative disease (DDD) is believed to originate in the nucleus pulposus (NP) region therefore, it is important to obtain a greater number of active NP cells for the study and therapy of DDD. Human induced pluripotent stem cells (iPSCs) are a powerful tool for modeling the development of DDD in humans, and have the potential to be applied in regenerative medicine. NP cells were isolated from DDD patients following our improved method, and then the primary NP cells were reprogramed into iPSCs with Sendai virus vectors encoding 4 factors. Successful reprogramming of iPSCs was verified by the expression of surface markers and presence of teratoma. Differentiation of iPSCs into NP-like cells was performed in a culture plate or in hydrogel, whereby skin fibroblast derived-iPSCs were used as a control. Results demonstrated that iPSCs derived from NP cells displayed a normal karyotype, expressed pluripotency markers, and formed teratoma in nude mice. NP induction of iPSCs resulted in the expression of NP cell specific matrix proteins and related genes. Non-induced NP derived-iPSCs also showed some NP-like phenotype. Furthermore, NP-derived iPSCs differentiate much better in hydrogel than that in a culture plate. This is a novel method for the generation of iPSCs from NP cells of DDD patients, and we have successfully differentiated these iPSCs into NP-like cells in hydrogel. This method provides a novel treatment of DDD by using patient-specific NP cells in a relatively simple and straightforward manner.
Keywords: differentiation; disc degenerative disease; induced pluripotent stem cell; nucleus pulposus; reprogram.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



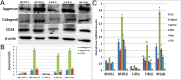
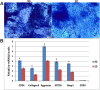
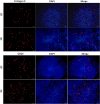
Similar articles
-
Differentiation of human induced pluripotent stem cells into nucleus pulposus-like cells.Stem Cell Res Ther. 2018 Mar 9;9(1):61. doi: 10.1186/s13287-018-0797-1. Stem Cell Res Ther. 2018. PMID: 29523190 Free PMC article.
-
Native nucleus pulposus tissue matrix promotes notochordal differentiation of human induced pluripotent stem cells with potential for treating intervertebral disc degeneration.J Biomed Mater Res A. 2015 Mar;103(3):1053-9. doi: 10.1002/jbm.a.35243. Epub 2014 Jun 17. J Biomed Mater Res A. 2015. PMID: 24889905
-
Human iPSCs can be differentiated into notochordal cells that reduce intervertebral disc degeneration in a porcine model.Theranostics. 2019 Oct 12;9(25):7506-7524. doi: 10.7150/thno.34898. eCollection 2019. Theranostics. 2019. PMID: 31695783 Free PMC article.
-
Differentiation of Pluripotent Stem Cells into Nucleus Pulposus Progenitor Cells for Intervertebral Disc Regeneration.Curr Stem Cell Res Ther. 2019;14(1):57-64. doi: 10.2174/1574888X13666180918095121. Curr Stem Cell Res Ther. 2019. PMID: 30227822 Review.
-
The embryonic and evolutionary boundaries between notochord and cartilage: a new look at nucleus pulposus-specific markers.Osteoarthritis Cartilage. 2018 Oct;26(10):1274-1282. doi: 10.1016/j.joca.2018.05.022. Epub 2018 Jun 21. Osteoarthritis Cartilage. 2018. PMID: 29935307 Review.
Cited by
-
Barriers to mesenchymal stromal cells for low back pain.World J Stem Cells. 2022 Dec 26;14(12):815-821. doi: 10.4252/wjsc.v14.i12.815. World J Stem Cells. 2022. PMID: 36619693 Free PMC article.
-
Concerns about cell therapy for intervertebral disc degeneration.NPJ Regen Med. 2022 Sep 6;7(1):46. doi: 10.1038/s41536-022-00245-4. NPJ Regen Med. 2022. PMID: 36068218 Free PMC article. No abstract available.
-
Cell-Based Therapies for Degenerative Musculoskeletal Diseases.Adv Sci (Weinh). 2023 Jul;10(21):e2207050. doi: 10.1002/advs.202207050. Epub 2023 May 18. Adv Sci (Weinh). 2023. PMID: 37199688 Free PMC article. Review.
-
Immortalized murine fibroblast cell lines are refractory to reprogramming to pluripotent state.Oncotarget. 2018 Oct 16;9(81):35241-35250. doi: 10.18632/oncotarget.26235. eCollection 2018 Oct 16. Oncotarget. 2018. PMID: 30443291 Free PMC article.
-
A 3-D hydrogel based system for hematopoietic differentiation and its use in modeling down syndrome associated transient myeloproliferative disorder.Biomater Sci. 2021 Sep 14;9(18):6266-6281. doi: 10.1039/d1bm00442e. Biomater Sci. 2021. PMID: 34369483 Free PMC article.
References
-
- Zhang Y, Xiong C, Chan C, Sakai D, Chan D. Changes in Nucleus Pulposus Cell Pools in “Healer” Mice for the Repair of Intervertebral Disc Degeneration. Global Spine J. 2015:05–P004.
-
- Lv F, Leung V, Cheung K. Cell-based Therapies for Degenerative Disc Diseases. Operative Techniques in Orthopaedics. 2016
-
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

