KDM1A/LSD1 regulates the differentiation and maintenance of spermatogonia in mice
- PMID: 28498828
- PMCID: PMC5428937
- DOI: 10.1371/journal.pone.0177473
KDM1A/LSD1 regulates the differentiation and maintenance of spermatogonia in mice
Abstract
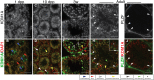
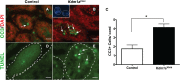
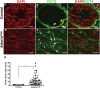
The proper regulation of spermatogenesis is crucial to ensure the continued production of sperm and fertility. Here, we investigated the function of the H3K4me2 demethylase KDM1A/LSD1 during spermatogenesis in developing and adult mice. Conditional deletion of Kdm1a in the testis just prior to birth leads to fewer spermatogonia and germ cell loss before 3 weeks of age. These results demonstrate that KDM1A is required for spermatogonial differentiation, as well as germ cell survival, in the developing testis. In addition, inducible deletion of Kdm1a in the adult testis results in the abnormal accumulation of meiotic spermatocytes, as well as apoptosis and progressive germ cell loss. These results demonstrate that KDM1A is also required during adult spermatogenesis. Furthermore, without KDM1A, the stem cell factor OCT4 is ectopically maintained in differentiating germ cells. This requirement for KDM1A is similar to what has been observed in other stem cell populations, suggesting a common function. Taken together, we propose that KDM1A is a key regulator of spermatogenesis and germ cell maintenance in the mouse.
Conflict of interest statement
Figures



References
-
- de Rooij DG. Proliferation and differentiation of spermatogonial stem cells. Reproduction. 2001;121(3):347–54. Epub 2001/02/28. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

