MeCP2 recognizes cytosine methylated tri-nucleotide and di-nucleotide sequences to tune transcription in the mammalian brain
- PMID: 28498846
- PMCID: PMC5446194
- DOI: 10.1371/journal.pgen.1006793
MeCP2 recognizes cytosine methylated tri-nucleotide and di-nucleotide sequences to tune transcription in the mammalian brain
Abstract
Mutations in the gene encoding the methyl-CG binding protein MeCP2 cause several neurological disorders including Rett syndrome. The di-nucleotide methyl-CG (mCG) is the classical MeCP2 DNA recognition sequence, but additional methylated sequence targets have been reported. Here we show by in vitro and in vivo analyses that MeCP2 binding to non-CG methylated sites in brain is largely confined to the tri-nucleotide sequence mCAC. MeCP2 binding to chromosomal DNA in mouse brain is proportional to mCAC + mCG density and unexpectedly defines large genomic domains within which transcription is sensitive to MeCP2 occupancy. Our results suggest that MeCP2 integrates patterns of mCAC and mCG in the brain to restrain transcription of genes critical for neuronal function.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


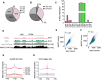

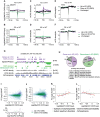
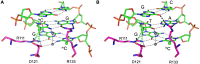
References
-
- Li E, Bestor TH, Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992;69: 915–26. Consulté: http://www.ncbi.nlm.nih.gov/pubmed/1606615 - PubMed
-
- Ramsahoye BH, Biniszkiewicz D, Lyko F, Clark V, Bird AP, Jaenisch R. Non-CpG methylation is prevalent in embryonic stem cells and may be mediated by DNA methyltransferase 3a. Proc Natl Acad Sci U S A. 2000;97: 5237–42. Consulté: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=25812&tool=pmc... - PMC - PubMed
-
- Aoki A, Suetake I, Miyagawa J, Fujio T, Chijiwa T, Sasaki H, et al. Enzymatic properties of de novo-type mouse DNA (cytosine-5) methyltransferases. Nucleic Acids Res. 2001;29: 3506–12. Consulté: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=55888&tool=pmc... - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

