Cost-effectiveness of pneumococcal vaccination strategies for the elderly in Korea
- PMID: 28498857
- PMCID: PMC5428995
- DOI: 10.1371/journal.pone.0177342
Cost-effectiveness of pneumococcal vaccination strategies for the elderly in Korea
Abstract
Background: Although the 13-valent pneumococcal conjugate vaccine (PCV13) showed good efficacy against pneumococcal disease in the the CAPiTA trial, the 23-valent pneumococcal polysaccharide vaccination (PPSV23) program has been ongoing for older adults aged ≥ 65 years in Korea since May of 2013. This study aimed to evaluate the cost-effectiveness of the current vaccination strategy (a single-dose PPSV23 vaccination) compared to a single-dose PCV13 vaccination and sequential PCV13-PPSV23 vaccinations in the elderly population aged ≥ 65 years.
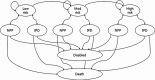
Methods: Using a Markov model, the incremental cost-effectiveness ratios (ICERs) of three vaccination strategies were assessed in a societal context. The transition probabilities, utility weights to estimate quality adjusted life year (QALY), and disease treatment costs were either calculated or cited from published data and the Health Insurance Review and Assessment Service. Simulations were performed in hypothetical cohorts of Korean adults aged ≥ 19 years. The vaccine effectiveness of PPSV23 was cited from a Cochrane Review report, while PCV13 effectiveness data were gathered from the CAPiTA trial.
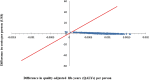
Results: Current PPSV23 vaccination strategies were cost-effective (ICER, $25,786 per QALY). However, the administration of PCV13 as a substitute for PPSV23 was shown to be more cost-effective than PPSV23 vaccination (ICER, $797 per QALY). Sequential PCV13-PPSV23 vaccination was also more cost-effective than PPSV23 for elderly people aged ≥ 65 years. In sensitivity analysis assuming significant PPSV23 effectiveness (50%) against non-bacteremic pneumococcal pneumonia, the PCV13 vaccination strategy was superior to the PPSV23 vaccination strategy in terms of cost-effectiveness.
Conclusion: The results suggest that PCV13 vaccination is more cost-effective in elderly subjects aged ≥ 65 years compared to the current PPSV23 vaccination strategy. When complete data is obtained in 2018 on the maximal herd effects of childhood PCV13 immunization, the incidence of pneumococcal pneumonia and the cost-effectiveness of vaccination strategies need to be reassessed.
Conflict of interest statement
Figures

Similar articles
-
Cost-effectiveness analysis of 13-valent pneumococcal conjugate vaccine versus 23-valent pneumococcal polysaccharide vaccine in an adult population in South Korea.Hum Vaccin Immunother. 2018;14(8):1914-1922. doi: 10.1080/21645515.2018.1456602. Epub 2018 Jul 11. Hum Vaccin Immunother. 2018. PMID: 29953307 Free PMC article.
-
Cost-effectiveness of adult vaccination strategies using pneumococcal conjugate vaccine compared with pneumococcal polysaccharide vaccine.JAMA. 2012 Feb 22;307(8):804-12. doi: 10.1001/jama.2012.169. JAMA. 2012. PMID: 22357831 Free PMC article.
-
Cost-effectiveness of the 13-valent pneumococcal conjugate vaccine in adults in Portugal versus "no vaccination" and versus vaccination with the 23-valent pneumococcal polysaccharide vaccine.Hum Vaccin Immunother. 2019;15(4):850-858. doi: 10.1080/21645515.2018.1560769. Epub 2019 Feb 20. Hum Vaccin Immunother. 2019. PMID: 30633615 Free PMC article.
-
Systematic literature review of cost-effectiveness analyses of adult 15- and 20-valent pneumococcal vaccines.Vaccine. 2025 Feb 6;46:126656. doi: 10.1016/j.vaccine.2024.126656. Epub 2024 Dec 27. Vaccine. 2025. PMID: 39731806
-
Cost Effectiveness of Elderly Pneumococcal Vaccination in Presence of Higher-Valent Pneumococcal Conjugate Childhood Vaccination: Systematic Literature Review with Focus on Methods and Assumptions.Pharmacoeconomics. 2019 Sep;37(9):1093-1127. doi: 10.1007/s40273-019-00805-5. Pharmacoeconomics. 2019. PMID: 31025189
Cited by
-
Response to Shami et al. 'Evaluating the cost-effectiveness of a sequential pneumococcal vaccination compared to single dose vaccination strategy for adults in Hong Kong' (Hum Vacc Immunother 2020).Hum Vaccin Immunother. 2021 Jan 2;17(1):173-175. doi: 10.1080/21645515.2020.1764828. Epub 2020 Jul 2. Hum Vaccin Immunother. 2021. PMID: 32614654 Free PMC article.
-
Continuous Effectiveness of Pneumococcal 13-Valent Conjugate Vaccine on Pediatric Pneumococcal Otomastoiditis: Results of 15 Years of Active/Prospective Surveillance in a Mexican Hospital on the Mexico-US Border.Cureus. 2021 Aug 31;13(8):e17608. doi: 10.7759/cureus.17608. eCollection 2021 Aug. Cureus. 2021. PMID: 34646659 Free PMC article.
-
Health and economic outcomes of 20-valent pneumococcal conjugate vaccine compared to 15-valent pneumococcal conjugate vaccine strategies for adults in Greece.Front Public Health. 2023 Sep 29;11:1229524. doi: 10.3389/fpubh.2023.1229524. eCollection 2023. Front Public Health. 2023. PMID: 37841729 Free PMC article.
-
Cost-Effectiveness of 20-Valent Pneumococcal Conjugate Vaccine in Argentinean Adults.Infect Dis Ther. 2024 Jun;13(6):1235-1251. doi: 10.1007/s40121-024-00972-9. Epub 2024 May 3. Infect Dis Ther. 2024. PMID: 38700655 Free PMC article.
-
The cost-effectiveness of using pneumococcal conjugate vaccine (PCV13) versus pneumococcal polysaccharide vaccine (PPSV23), in South African adults.PLoS One. 2020 Jan 29;15(1):e0227945. doi: 10.1371/journal.pone.0227945. eCollection 2020. PLoS One. 2020. PMID: 31995597 Free PMC article.
References
-
- Moberley SA, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2008;(1):Cd000422 Epub 2008/02/07. doi: 10.1002/14651858.CD000422.pub2 - DOI - PubMed
-
- Diao WQ, Shen N, Yu PX, Liu BB, He B. Efficacy of 23-valent pneumococcal polysaccharide vaccine in preventing community-acquired pneumonia among immunocompetent adults: A systematic review and meta-analysis of randomized trials. Vaccine. 2016;34(13):1496–503. Epub 2016/02/24. doi: 10.1016/j.vaccine.2016.02.023 - DOI - PubMed
-
- Huss A, Scott P, Stuck AE, Trotter C, Egger M. Efficacy of pneumococcal vaccination in adults: a meta-analysis. CMAJ. 2009;180(1):48–58. Epub 2009/01/07. PubMed Central PMCID: PMCPMC2612051. doi: 10.1503/cmaj.080734 - DOI - PMC - PubMed
-
- Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2013;(1):Cd000422 Epub 2013/02/27. doi: 10.1002/14651858.CD000422.pub3 - DOI - PMC - PubMed
-
- Bonten MJ, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372(12):1114–25. Epub 2015/03/19. doi: 10.1056/NEJMoa1408544 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

