Incident AIDS or Death After Initiation of Human Immunodeficiency Virus Treatment Regimens Including Raltegravir or Efavirenz Among Adults in the United States
- PMID: 28498892
- PMCID: PMC6059161
- DOI: 10.1093/cid/cix199
Incident AIDS or Death After Initiation of Human Immunodeficiency Virus Treatment Regimens Including Raltegravir or Efavirenz Among Adults in the United States
Erratum in
-
Erratum.Clin Infect Dis. 2017 Oct 15;65(8):1431-1433. doi: 10.1093/cid/cix563. Clin Infect Dis. 2017. PMID: 29017252 Free PMC article. No abstract available.
Abstract
Background.: The long-term effectiveness of human immunodeficiency virus (HIV) treatments containing integrase inhibitors is unknown.
Methods.: We use observational data from the Centers for AIDS Research Network of Integrated Clinical Systems and the Centers for Disease Control and Prevention to estimate 4-year risk of AIDS and all-cause mortality among 415 patients starting a raltegravir regimen compared to 2646 starting an efavirenz regimen (both regimens include emtricitabine and tenofovir disoproxil fumarate). We account for confounding and selection bias as well as generalizability by standardization for measured variables, and present both observational intent-to-treat and per-protocol estimates.
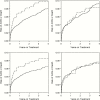
Results.: At treatment initiation, 12% of patients were female, 36% black, 13% Hispanic; median age was 37 years, CD4 count 321 cells/µL, and viral load 4.5 log10 copies/mL. Two hundred thirty-five patients incurred an AIDS-defining illness or died, and 741 patients left follow-up. After accounting for measured differences, the 4-year risk was similar among those starting both regimens (ie, intent-to treat hazard ratio [HR], 0.96 [95% confidence interval {CI}, .63-1.45]; risk difference, -0.9 [95% CI, -4.5 to 2.7]), as well as among those remaining on regimens (ie, per-protocol HR, 0.95 [95% CI, .59-1.54]; risk difference, -0.5 [95% CI, -3.8 to 2.9]).
Conclusions.: Raltegravir and efavirenz-based initial antiretroviral therapy have similar 4-year clinical effects. Vigilance regarding longer-term comparative effectiveness of HIV regimens using observational data is needed because large-scale experimental data are not forthcoming.
Keywords: HIV; cohort study; comparative effectiveness; mortality.; raltegravir.
© The Author 2017. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
References
-
- Lennox JL, DeJesus E, Lazzarin A et al. ; STARTMRK investigators Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet 2009; 374:796–806. - PubMed
-
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Atlanta, GA: Centers for Disease Control and Prevention, 2016.
-
- Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas, 2013. HIV Surveillance Supplemental Report 20 (No. 2). Atlanta, GA: CDC, 2015.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

