Proteolytic cleavage is required for functional neuroligin 2 maturation and trafficking in Drosophila
- PMID: 28498949
- PMCID: PMC5907836
- DOI: 10.1093/jmcb/mjx015
Proteolytic cleavage is required for functional neuroligin 2 maturation and trafficking in Drosophila
Abstract
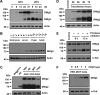
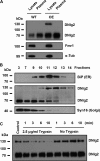
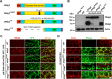
Neuroligins (Nlgs) are transmembrane cell adhesion molecules playing essential roles in synapse development and function. Genetic mutations in neuroligin genes have been linked with some neurodevelopmental disorders such as autism. These mutated Nlgs are mostly retained in the endoplasmic reticulum (ER). However, the mechanisms underlying normal Nlg maturation and trafficking have remained largely unknown. Here, we found that Drosophila neuroligin 2 (DNlg2) undergoes proteolytic cleavage in the ER in a variety of Drosophila tissues throughout developmental stages. A region encompassing Y642-T698 is required for this process. The immature non-cleavable DNlg2 is retained in the ER and non-functional. The C-terminal fragment of DNlg2 instead of the full-length or non-cleavable DNlg2 is able to rescue neuromuscular junction defects and GluRIIB reduction induced by dnlg2 deletion. Intriguingly, the autism-associated R598C mutation in DNlg2 leads to similar marked defects in DNlg2 proteolytic process and ER export, revealing a potential role of the improper Nlg cleavage in autism pathogenesis. Collectively, our findings uncover a specific mechanism that controls DNlg2 maturation and trafficking via proteolytic cleavage in the ER, suggesting that the perturbed proteolytic cleavage of Nlgs likely contributes to autism disorder.
Keywords: autism; maturation; neuroligin; proteolytic cleavage; trafficking.
© The Author (2017). Published by Oxford University Press on behalf of Journal of Molecular Cell Biology, IBCB, SIBS, CAS.
Figures




Similar articles
-
Drosophila neuroligin 2 is required presynaptically and postsynaptically for proper synaptic differentiation and synaptic transmission.J Neurosci. 2012 Nov 7;32(45):16018-30. doi: 10.1523/JNEUROSCI.1685-12.2012. J Neurosci. 2012. PMID: 23136438 Free PMC article.
-
Proteolytic maturation of Drosophila Neuroligin 3 by tumor necrosis factor α-converting enzyme in the nervous system.Biochim Biophys Acta Gen Subj. 2018 Mar;1862(3):440-450. doi: 10.1016/j.bbagen.2017.10.021. Epub 2017 Oct 28. Biochim Biophys Acta Gen Subj. 2018. PMID: 29107812
-
Neuroligin 4 regulates synaptic growth via the bone morphogenetic protein (BMP) signaling pathway at the Drosophila neuromuscular junction.J Biol Chem. 2017 Nov 3;292(44):17991-18005. doi: 10.1074/jbc.M117.810242. Epub 2017 Sep 14. J Biol Chem. 2017. PMID: 28912273 Free PMC article.
-
[Synapse maturation and autism: learning from neuroligin model mice].Nihon Shinkei Seishin Yakurigaku Zasshi. 2014 Feb;34(1):1-4. Nihon Shinkei Seishin Yakurigaku Zasshi. 2014. PMID: 25069265 Review. Japanese.
-
Genetic factors and epigenetic factors for autism: endoplasmic reticulum stress and impaired synaptic function.Cell Biol Int. 2009 Dec 16;34(1):13-9. doi: 10.1042/CBI20090250. Cell Biol Int. 2009. PMID: 20001973 Review.
Cited by
-
Neurexin-Neuroligin 1 regulates synaptic morphology and functions via the WAVE regulatory complex in Drosophila neuromuscular junction.Elife. 2018 Mar 14;7:e30457. doi: 10.7554/eLife.30457. Elife. 2018. PMID: 29537369 Free PMC article.
-
Short Time-Series Expression Transcriptome Data Reveal the Gene Expression Patterns of Dairy Cow Mammary Gland as Milk Yield Decreased Process.Genes (Basel). 2021 Jun 20;12(6):942. doi: 10.3390/genes12060942. Genes (Basel). 2021. PMID: 34203058 Free PMC article.
-
Neuroligin 1, 2, and 3 Regulation at the Synapse: FMRP-Dependent Translation and Activity-Induced Proteolytic Cleavage.Mol Neurobiol. 2019 Apr;56(4):2741-2759. doi: 10.1007/s12035-018-1243-1. Epub 2018 Jul 28. Mol Neurobiol. 2019. PMID: 30056576 Free PMC article.
-
Neuroligin 2 governs synaptic morphology and function through RACK1-cofilin signaling in Drosophila.Commun Biol. 2023 Oct 18;6(1):1056. doi: 10.1038/s42003-023-05428-3. Commun Biol. 2023. PMID: 37853189 Free PMC article.
References
-
- Banovic D., Khorramshahi O., Owald D., et al. (2010). Drosophila neuroligin 1 promotes growth and postsynaptic differentiation at glutamatergic neuromuscular junctions. Neuron 66, 724–738. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

