Strong transcription blockage mediated by R-loop formation within a G-rich homopurine-homopyrimidine sequence localized in the vicinity of the promoter
- PMID: 28498974
- PMCID: PMC5499740
- DOI: 10.1093/nar/gkx403
Strong transcription blockage mediated by R-loop formation within a G-rich homopurine-homopyrimidine sequence localized in the vicinity of the promoter
Abstract
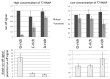
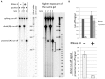
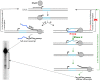
Guanine-rich (G-rich) homopurine-homopyrimidine nucleotide sequences can block transcription with an efficiency that depends upon their orientation, composition and length, as well as the presence of negative supercoiling or breaks in the non-template DNA strand. We report that a G-rich sequence in the non-template strand reduces the yield of T7 RNA polymerase transcription by more than an order of magnitude when positioned close (9 bp) to the promoter, in comparison to that for a distal (∼250 bp) location of the same sequence. This transcription blockage is much less pronounced for a C-rich sequence, and is not significant for an A-rich sequence. Remarkably, the blockage is not pronounced if transcription is performed in the presence of RNase H, which specifically digests the RNA strands within RNA-DNA hybrids. The blockage also becomes less pronounced upon reduced RNA polymerase concentration. Based upon these observations and those from control experiments, we conclude that the blockage is primarily due to the formation of stable RNA-DNA hybrids (R-loops), which inhibit successive rounds of transcription. Our results could be relevant to transcription dynamics in vivo (e.g. transcription 'bursting') and may also have practical implications for the design of expression vectors.
© The Author(s) 2017. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



Similar articles
-
Transcription blockage by homopurine DNA sequences: role of sequence composition and single-strand breaks.Nucleic Acids Res. 2013 Feb 1;41(3):1817-28. doi: 10.1093/nar/gks1333. Epub 2012 Dec 28. Nucleic Acids Res. 2013. PMID: 23275544 Free PMC article.
-
Mechanisms and implications of transcription blockage by guanine-rich DNA sequences.Proc Natl Acad Sci U S A. 2010 Jul 20;107(29):12816-21. doi: 10.1073/pnas.1007580107. Epub 2010 Jun 28. Proc Natl Acad Sci U S A. 2010. PMID: 20616059 Free PMC article.
-
PNA binding to the non-template DNA strand interferes with transcription, suggesting a blockage mechanism mediated by R-loop formation.Mol Carcinog. 2015 Nov;54(11):1508-12. doi: 10.1002/mc.22209. Epub 2014 Aug 30. Mol Carcinog. 2015. PMID: 25175074 Free PMC article.
-
Competition between the RNA transcript and the nontemplate DNA strand during R-loop formation in vitro: a nick can serve as a strong R-loop initiation site.Mol Cell Biol. 2010 Jan;30(1):146-59. doi: 10.1128/MCB.00897-09. Mol Cell Biol. 2010. PMID: 19841062 Free PMC article.
-
Structure and function in promoter escape by T7 RNA polymerase.Prog Nucleic Acid Res Mol Biol. 2005;80:323-47. doi: 10.1016/S0079-6603(05)80008-X. Prog Nucleic Acid Res Mol Biol. 2005. PMID: 16164978 Review. No abstract available.
Cited by
-
Genome-wide relationship between R-loop formation and antisense transcription in Escherichia coli.Nucleic Acids Res. 2018 Apr 20;46(7):3400-3411. doi: 10.1093/nar/gky118. Nucleic Acids Res. 2018. PMID: 29474582 Free PMC article.
-
A novel mode for transcription inhibition mediated by PNA-induced R-loops with a model in vitro system.Biochim Biophys Acta Gene Regul Mech. 2018 Feb;1861(2):158-166. doi: 10.1016/j.bbagrm.2017.12.008. Epub 2018 Jan 31. Biochim Biophys Acta Gene Regul Mech. 2018. PMID: 29357316 Free PMC article.
-
G-quadruplex DNA: a novel target for drug design.Cell Mol Life Sci. 2021 Oct;78(19-20):6557-6583. doi: 10.1007/s00018-021-03921-8. Epub 2021 Aug 30. Cell Mol Life Sci. 2021. PMID: 34459951 Free PMC article. Review.
-
Defining R-loop classes and their contributions to genome instability.DNA Repair (Amst). 2021 Oct;106:103182. doi: 10.1016/j.dnarep.2021.103182. Epub 2021 Jul 17. DNA Repair (Amst). 2021. PMID: 34303066 Free PMC article. Review.
-
Transcription-Replication Collisions-A Series of Unfortunate Events.Biomolecules. 2021 Aug 21;11(8):1249. doi: 10.3390/biom11081249. Biomolecules. 2021. PMID: 34439915 Free PMC article. Review.
References
-
- Belotserkovskii B.P., Mirkin S.M., Hanawalt P.C.. DNA sequences that interfere with transcription: implications for genome function and stability. Chem. Rev. 2013; 113:8620–8637. - PubMed
-
- Aguilera A., Garcia-Muse T.. R loops: from transcription byproducts to threats to genome stability. Mol. Cell. 2012; 46:115–124. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

