Sustained efficacy, safety and patient-reported outcomes of certolizumab pegol in axial spondyloarthritis: 4-year outcomes from RAPID-axSpA
- PMID: 28498975
- PMCID: PMC5850296
- DOI: 10.1093/rheumatology/kex174
Sustained efficacy, safety and patient-reported outcomes of certolizumab pegol in axial spondyloarthritis: 4-year outcomes from RAPID-axSpA
Abstract
Objective: The aim was to assess the long-term safety and efficacy of certolizumab pegol over 4 years of continuous treatment in patients with axial spondyloarthritis (axSpA), including both AS and non-radiographic (nr-) axSpA.
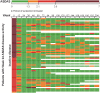
Methods: RAPID-axSpA was a phase 3 randomized trial, double blind and placebo controlled to week 24, dose blind to week 48 and open label to week 204. Patients had a clinical diagnosis of axSpA, meeting Assessment of SpondyloArthritis international Society (ASAS) criteria, and had active disease. The assessed outcomes included ASAS20, ASAS40, AS DAS (ASDAS), BASDAI, BASFI and BASMI scores, along with selected measures of remission. Further patient-reported outcomes, peripheral arthritis, enthesitis, uveitis and quality-of-life measures are also reported.
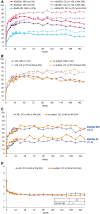
Results: Two hundred and eighteen of 325 patients randomized (AS: 121; nr-axSpA: 97) received certolizumab pegol from week 0. Of these, 65% remained in the study at week 204 (AS: 67%; nr-axSpA: 63%). Across all outcomes, for AS and nr-axSpA, sustained improvements were observed to week 204 [week 204 overall axSpA: ASAS20: 54.1% (non-responder imputation); 83.7% (observed case, OC); ASAS40: 44.0% (non-responder imputation); 68.1% (OC); ASDAS inactive disease: 32.1% (last observation carried forward); 31.4% (OC)]. In the safety set (n = 315), there were 292.8 adverse events and 10.4 serious adverse events per 100 patient-years. No deaths were reported.
Conclusion: In the first study to evaluate the efficacy of an anti-TNF across both axSpA subpopulations, improvements in clinical and patient-reported outcomes at 24 and 96 weeks were sustained through 4 years of treatment, with no new safety signals.
Trial registration: ClinicalTrials.gov, http://clinicaltrials.gov, NCT01087762.
Keywords: ankylosing spondylitis; axial spondyloarthritis; certolizumab pegol; non-radiographic axial spondyloarthritis.
© The Author 2017. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures

References
-
- Linden SVD, Valkenburg HA, Cats A.. Evaluation of diagnostic criteria for ankylosing spondylitis. Arthritis Rheum 1984;27:361–8. - PubMed
-
- Rudwaleit M, van der Heijde D, Landewe R. et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83. - PubMed
-
- Stolwijk C, van Tubergen A, Castillo-Ortiz JD, Boonen A.. Prevalence of extra-articular manifestations in patients with ankylosing spondylitis: a systematic review and meta-analysis. Ann Rheum Dis 2015;74:65–73. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

