Consequences of MEGF10 deficiency on myoblast function and Notch1 interactions
- PMID: 28498977
- PMCID: PMC6075367
- DOI: 10.1093/hmg/ddx189
Consequences of MEGF10 deficiency on myoblast function and Notch1 interactions
Abstract
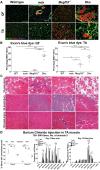
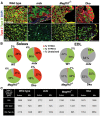
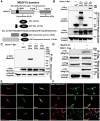
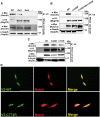
Mutations in MEGF10 cause early onset myopathy, areflexia, respiratory distress, and dysphagia (EMARDD), a rare congenital muscle disease, but the pathogenic mechanisms remain largely unknown. We demonstrate that short hairpin RNA (shRNA)-mediated knockdown of Megf10, as well as overexpression of the pathogenic human p.C774R mutation, leads to impaired proliferation and migration of C2C12 cells. Myoblasts from Megf10-/- mice and Megf10-/-/mdx double knockout (dko) mice also show impaired proliferation and migration compared to myoblasts from wild type and mdx mice, whereas the dko mice show histological abnormalities that are not observed in either single mutant mouse. Cell proliferation and migration are known to be regulated by the Notch receptor, which plays an essential role in myogenesis. Reciprocal co-immunoprecipitation studies show that Megf10 and Notch1 interact via their respective intracellular domains. These interactions are impaired by the pathogenic p.C774R mutation. Megf10 regulation of myoblast function appears to be mediated at least in part via interactions with key components of the Notch signaling pathway, and defects in these interactions may contribute to the pathogenesis of EMARDD.
© The Author 2017. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures






Similar articles
-
Selective serotonin reuptake inhibitors ameliorate MEGF10 myopathy.Hum Mol Genet. 2019 Jul 15;28(14):2365-2377. doi: 10.1093/hmg/ddz064. Hum Mol Genet. 2019. PMID: 31267131 Free PMC article.
-
Cysteine mutations cause defective tyrosine phosphorylation in MEGF10 myopathy.FEBS Lett. 2013 Sep 17;587(18):2952-7. doi: 10.1016/j.febslet.2013.08.002. Epub 2013 Aug 15. FEBS Lett. 2013. PMID: 23954233 Free PMC article.
-
The impact of Megf10/Drpr gain-of-function on muscle development in Drosophila.FEBS Lett. 2019 Apr;593(7):680-696. doi: 10.1002/1873-3468.13348. Epub 2019 Mar 12. FEBS Lett. 2019. PMID: 30802937 Free PMC article.
-
MEGF10 related myopathies: A new case with adult onset disease with prominent respiratory failure and review of reported phenotypes.Neuromuscul Disord. 2018 Jan;28(1):48-53. doi: 10.1016/j.nmd.2017.09.017. Epub 2017 Oct 12. Neuromuscul Disord. 2018. PMID: 29128256 Review.
-
[A family with early onset myopathy caused by MEGF10 gene defect and literature review].Zhonghua Er Ke Za Zhi. 2023 Mar 2;61(3):261-265. doi: 10.3760/cma.j.cn112140-20221214-01046. Zhonghua Er Ke Za Zhi. 2023. PMID: 36849355 Review. Chinese.
Cited by
-
The role of MEGF10 in myoblast fusion and hypertrophic response to overload of skeletal muscle.J Muscle Res Cell Motil. 2025 Jan 18. doi: 10.1007/s10974-024-09686-4. Online ahead of print. J Muscle Res Cell Motil. 2025. PMID: 39825147
-
Current Thoughts of Notch's Role in Myoblast Regulation and Muscle-Associated Disease.Int J Environ Res Public Health. 2021 Nov 29;18(23):12558. doi: 10.3390/ijerph182312558. Int J Environ Res Public Health. 2021. PMID: 34886282 Free PMC article. Review.
-
Molecular characterisation of osteoblasts from bone obtained from people of Polynesian and European ancestry undergoing joint replacement surgery.Sci Rep. 2021 Jan 28;11(1):2428. doi: 10.1038/s41598-021-81731-5. Sci Rep. 2021. PMID: 33510208 Free PMC article.
-
Implications of notch signaling in duchenne muscular dystrophy.Front Physiol. 2022 Sep 27;13:984373. doi: 10.3389/fphys.2022.984373. eCollection 2022. Front Physiol. 2022. PMID: 36237531 Free PMC article. Review.
-
The Notch signaling network in muscle stem cells during development, homeostasis, and disease.Skelet Muscle. 2022 Apr 22;12(1):9. doi: 10.1186/s13395-022-00293-w. Skelet Muscle. 2022. PMID: 35459219 Free PMC article. Review.
References
-
- Suzuki E., Nakayama M. (2007) The mammalian Ced-1 ortholog MEGF10/KIAA1780 displays a novel adhesion pattern. Exp. Cell Res., 313, 2451–2464. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

