The association of hormone signalling genes, transcription and changes in shoot anatomy during moso bamboo growth
- PMID: 28499069
- PMCID: PMC5785349
- DOI: 10.1111/pbi.12750
The association of hormone signalling genes, transcription and changes in shoot anatomy during moso bamboo growth
Abstract
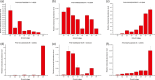
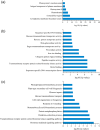
Moso bamboo is a large, woody bamboo with the highest ecological, economic and cultural value of all the bamboo types and accounts for up to 70% of the total area of bamboo grown. However, the spatiotemporal variation role of moso bamboo shoot during growth period is still unclear. We found that the bamboo shoot growth can be divided into three distinct periods, including winter growth, early growth and late growth based on gene expression and anatomy. In the early growth period, lateral buds germinated from the top of the bamboo joint in the shoot tip. Intercalary meristems grew vigorously during the winter growth period and early growth period, but in the late growth period, mitosis in the intercalary meristems decreased. The expression of cell cycle-associated genes and the quantity of differentially expressed genes were higher in early growth than those in late growth, appearing to be influenced by hormonal concentrations. Gene expression analysis indicates that hormone signalling genes play key roles in shoot growth, while auxin signalling genes play a central role. In situ hybridization analyses illustrate how auxin signalling genes regulate apical dominance, meristem maintenance and lateral bud development. Our study provides a vivid picture of the dynamic changes in anatomy and gene expression during shoot growth in moso bamboo, and how hormone signalling-associated genes participate in moso bamboo shoot growth.
Keywords: endogenous hormone; fast growth; hormone signalling genes; moso bamboo; shoot anatomy.
© 2017 The Authors. Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Conflict of interest statement
All the authors have declared no conflict of interest.
Figures










Similar articles
-
Genome-wide analysis of shoot growth-associated alternative splicing in moso bamboo.Mol Genet Genomics. 2016 Aug;291(4):1695-714. doi: 10.1007/s00438-016-1212-1. Epub 2016 May 11. Mol Genet Genomics. 2016. PMID: 27170010
-
Uncovering PheCLE1 and PheCLE10 Promoting Root Development Based on Genome-Wide Analysis.Int J Mol Sci. 2024 Jun 29;25(13):7190. doi: 10.3390/ijms25137190. Int J Mol Sci. 2024. PMID: 39000298 Free PMC article.
-
PheGRF4e initiated auxin signaling during moso bamboo shoot development.Mol Biol Rep. 2022 Sep;49(9):8815-8825. doi: 10.1007/s11033-022-07731-4. Epub 2022 Jul 22. Mol Biol Rep. 2022. PMID: 35867290
-
Transcriptomic Complexity of Culm Growth and Development in Different Types of Moso Bamboo.Int J Mol Sci. 2023 Apr 18;24(8):7425. doi: 10.3390/ijms24087425. Int J Mol Sci. 2023. PMID: 37108588 Free PMC article. Review.
-
Shoot and inflorescence branching.Curr Opin Plant Biol. 2005 Oct;8(5):506-11. doi: 10.1016/j.pbi.2005.07.010. Curr Opin Plant Biol. 2005. PMID: 16054429 Review.
Cited by
-
Single-cell transcriptome atlas reveals spatiotemporal developmental trajectories in the basal roots of moso bamboo (Phyllostachys edulis).Hortic Res. 2023 Jun 9;10(8):uhad122. doi: 10.1093/hr/uhad122. eCollection 2023 Aug. Hortic Res. 2023. PMID: 37554343 Free PMC article.
-
GA20ox Family Genes Mediate Gibberellin and Auxin Crosstalk in Moso bamboo (Phyllostachys edulis).Plants (Basel). 2023 Aug 1;12(15):2842. doi: 10.3390/plants12152842. Plants (Basel). 2023. PMID: 37570996 Free PMC article.
-
Association among starch storage, metabolism, related genes and growth of Moso bamboo (Phyllostachys heterocycla) shoots.BMC Plant Biol. 2021 Oct 20;21(1):477. doi: 10.1186/s12870-021-03257-2. BMC Plant Biol. 2021. PMID: 34670492 Free PMC article.
-
Genome-wide identification and expression analysis of brassinosteroid action-related genes during the shoot growth of moso bamboo.Mol Biol Rep. 2019 Apr;46(2):1909-1930. doi: 10.1007/s11033-019-04642-9. Epub 2019 Feb 5. Mol Biol Rep. 2019. PMID: 30721422
-
Culm Morphological Analysis in Moso Bamboo Reveals the Negative Regulation of Internode Diameter and Thickness by Monthly Precipitation.Plants (Basel). 2024 May 28;13(11):1484. doi: 10.3390/plants13111484. Plants (Basel). 2024. PMID: 38891293 Free PMC article.
References
-
- Ali, M. , Brian, A.W. , Kenneth, M. , Lorian, S. and Barbara, W. (2008) Mapping and quantifying mammalian transcriptomes by RNA‐Seq. Nat. Methods, 5, 621–628. - PubMed
-
- Cui, K. , He, C.Y. , Zhang, J.G. , Duan, A.G. and Zeng, Y.F. (2012) Temporal and spatial profiling of internode elongation‐associated protein expression in rapidly growing culms of bamboo. J. Proteome Res. 11, 2492–2507. - PubMed
Publication types
MeSH terms
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

