Identification of Intrahelical Bifurcated H-Bonds as a New Type of Gate in K+ Channels
- PMID: 28499087
- PMCID: PMC6638992
- DOI: 10.1021/jacs.7b01158
Identification of Intrahelical Bifurcated H-Bonds as a New Type of Gate in K+ Channels
Abstract
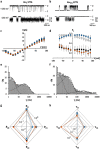
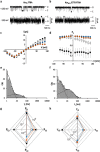
Gating of ion channels is based on structural transitions between open and closed states. To uncover the chemical basis of individual gates, we performed a comparative experimental and computational analysis between two K+ channels, KcvS and KcvNTS. These small viral encoded K+ channel proteins, with a monomer size of only 82 amino acids, resemble the pore module of all complex K+ channels in terms of structure and function. Even though both proteins share about 90% amino acid sequence identity, they exhibit different open probabilities with ca. 90% in KcvNTS and 40% in KcvS. Single channel analysis, mutational studies and molecular dynamics simulations show that the difference in open probability is caused by one long closed state in KcvS. This state is structurally created in the tetrameric channel by a transient, Ser mediated, intrahelical hydrogen bond. The resulting kink in the inner transmembrane domain swings the aromatic rings from downstream Phes in the cavity of the channel, which blocks ion flux. The frequent occurrence of Ser or Thr based helical kinks in membrane proteins suggests that a similar mechanism could also occur in the gating of other ion channels.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Hille B.Ion Channels of Excitable Membranes, 3rd ed.; Sinauer Associates Inc.: Sunderland, 2001.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

