Probing for Neuroadaptations to Unpredictable Stressors in Addiction: Translational Methods and Emerging Evidence
- PMID: 28499100
- PMCID: PMC5440361
- DOI: 10.15288/jsad.2017.78.353
Probing for Neuroadaptations to Unpredictable Stressors in Addiction: Translational Methods and Emerging Evidence
Abstract
Stressors clearly contribute to addiction etiology and relapse in humans, but our understanding of specific mechanisms remains limited. Rodent models of addiction offer the power, flexibility, and precision necessary to delineate the causal role and specific mechanisms through which stressors influence alcohol and other drug use. This review describes a program of research using startle potentiation to unpredictable stressors that is well positioned to translate between animal models and clinical research with humans on stress neuroadaptations in addiction. This research rests on a solid foundation provided by three separate pillars of evidence from (a) rodent behavioral neuroscience on stress neuroadaptations in addiction, (b) rodent affective neuroscience on startle potentiation, and (c) human addiction and affective science with startle potentiation. Rodent stress neuroadaptation models implicate adaptations in corticotropin-releasing factor and norepinephrine circuits within the central extended amygdala following chronic alcohol and other drug use that mediate anxious behaviors and stress-induced reinstatement among drug-dependent rodents. Basic affective neuroscience indicates that these same neural mechanisms are involved in startle potentiation to unpredictable stressors in particular (vs. predictable stressors). We believe that synthesis of these evidence bases should focus us on the role of unpredictable stressors in addiction etiology and relapse. Startle potentiation in unpredictable stressor tasks is proposed to provide an attractive and flexible test bed to encourage tight translation and reverse translation between animal models and human clinical research on stress neuroadaptations. Experimental therapeutics approaches focused on unpredictable stressors hold high promise to identify, repurpose, or refine pharmacological and psychosocial interventions for addiction.
Figures

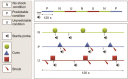
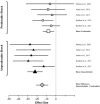
Comment in
-
Targeting Stress Neuroadaptations for Addiction Treatment: A Commentary on Kaye et al. (2017).J Stud Alcohol Drugs. 2017 May;78(3):372-374. doi: 10.15288/jsad.2017.78.372. J Stud Alcohol Drugs. 2017. PMID: 28499101 Free PMC article. No abstract available.
References
-
- Ahmed S. H., Walker J. R., Koob G. F. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22:413–421. doi:10.1016/S0893-133X(99)00133-5. - PubMed
-
- Al’Absi M. Hypothalamic-pituitary-adrenocortical responses to psychological stress and risk for smoking relapse. International Journal of Psychophysiology. 2006;59:218–227. doi:10.1016/j.ijpsycho.2005.10.010. - PubMed
-
- Alheid G. F., Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: The striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. - PubMed
-
- Amato L., Davoli M., Perucci C. A., Ferri M., Faggiano F., Mattick R. P. An overview of systematic reviews of the effectiveness of opiate maintenance therapies: Available evidence to inform clinical practice and research. Journal of Substance Abuse Treatment. 2005;28:321–329. doi:10.1016/j.jsat.2005.02.007. - PubMed

