Is hydroxychloroquine effective in treating primary Sjogren's syndrome: a systematic review and meta-analysis
- PMID: 28499370
- PMCID: PMC5427554
- DOI: 10.1186/s12891-017-1543-z
Is hydroxychloroquine effective in treating primary Sjogren's syndrome: a systematic review and meta-analysis
Abstract
Background: To systematically review and assess the efficacy and safety of hydroxychloroquine (HCQ) for treating primary Sjogren's syndrome (pSS).
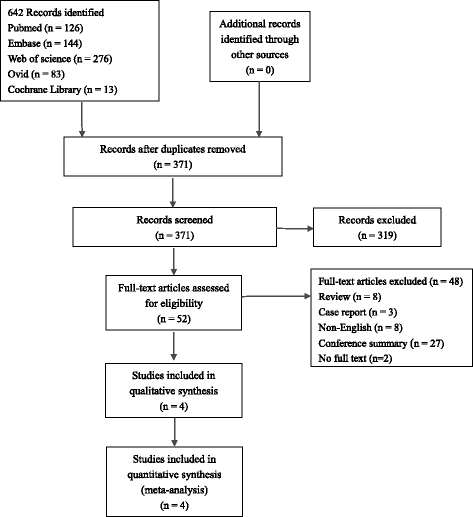
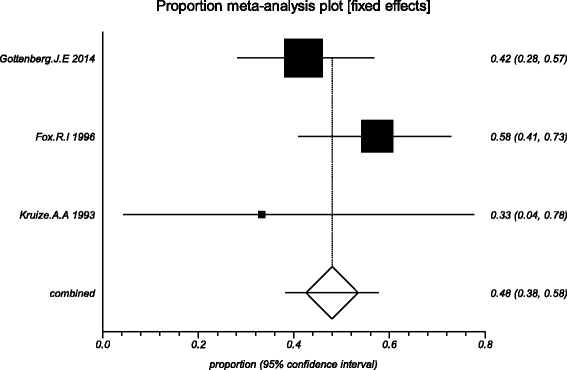
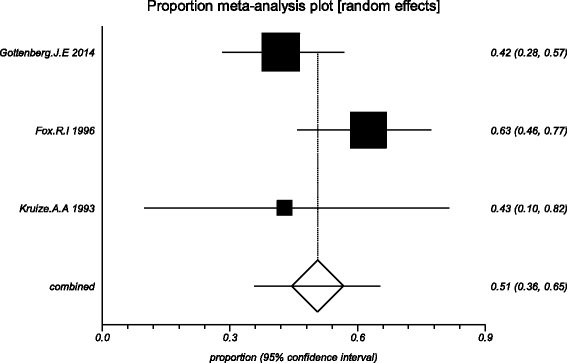
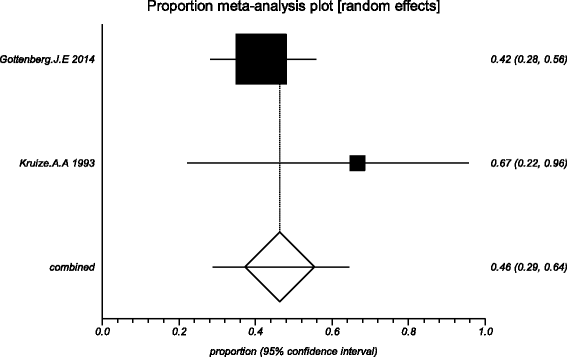
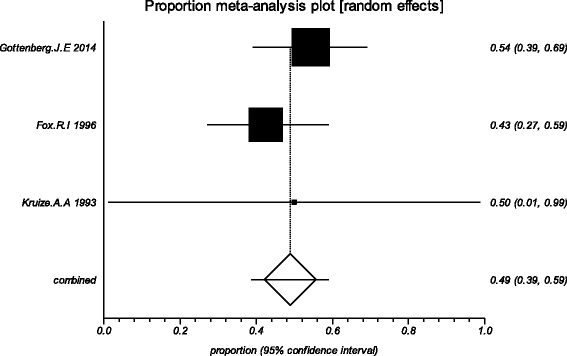
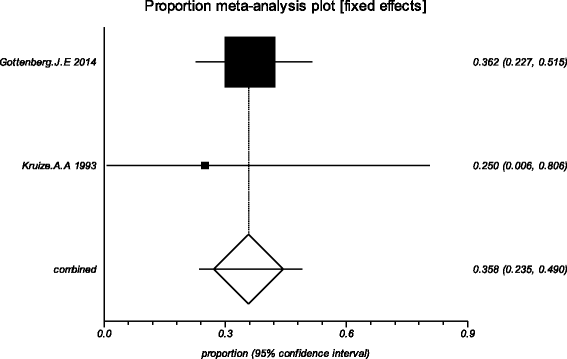
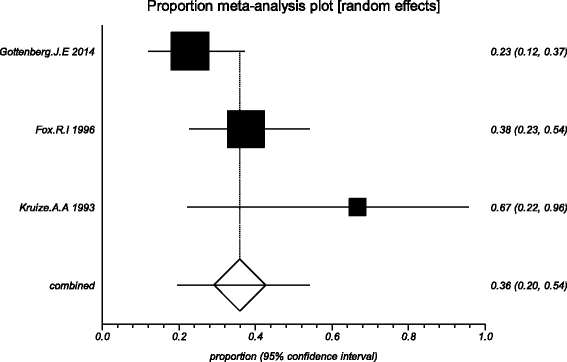
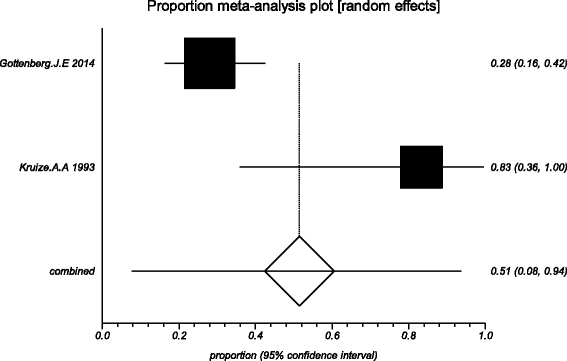
Methods: Five electronic databases (Pubmed, EMBASE, Web of science, Ovid, Cochrane Library) were searched for randomized controlled trials and retrospective or prospective studies published in English that reported the effect of HCQ on pSS. The subjective symptoms (sicca symptoms, fatigue and pain) and the objective indexes (erythrocyte sedimentation rate and Schirmer test) were assessed as main outcome measures. A meta-analysis and descriptive study on the efficacy and safety of HCQ were conducted. The estimate of the effect of HCQ treatment was expressed as a proportion together with 95% confidence interval, and plotted on a forest plot.
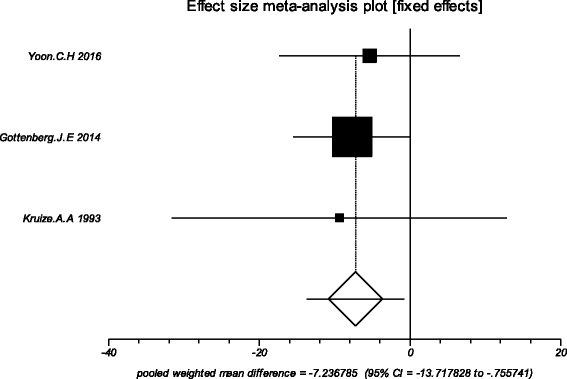
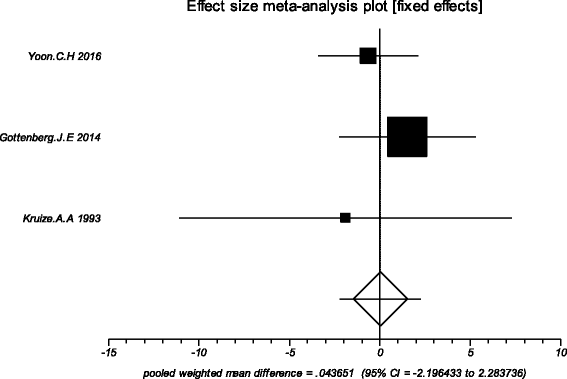
Results: Four trials with totals of 215 SS patients, including two randomized controlled trials, one double blind crossover trial and one retrospective open-label study, were analyzed in this review. For dry mouth and dry eyes, the effectiveness of HCQ treatment was essentially the same as placebo treatment. For fatigue, the effectiveness of HCQ was lower than placebo. The efficacy of HCQ in treating pain associated with pSS was superior to that of the placebo. There was no significant difference between HCQ-treated groups and controls in terms of Schirmer test results, but HCQ could reduce the erythrocyte sedimentation rate compare with placebo. A descriptive safety assessment showed that gastrointestinal adverse effects were the most common adverse effects associated with HCQ.
Conclusions: This systematic review showed that there is no significant difference between HCQ and placebo in the treatment of dry mouth and dry eye in pSS. Well-designed, randomized, controlled trials are needed to provide higher-quality evidence to confirm our findings, and future studies should focus on some other index or extraglandular measures, such as cutaneous manifestations, to further explore the therapeutic effect of HCQ in pSS.
Keywords: Hydroxychloroquine; Meta-analysis; Sjogren’s syndrome; Systematic review.
Figures

References
-
- ter Borg EJ, Risselada AP, Kelder JC. Relation of systemic autoantibodies to the number of extraglandular manifestations in primary Sjogren’s Syndrome: a retrospective analysis of 65 patients in the Netherlands. Semin Arthritis Rheum. 2011;40(6):547–551. doi: 10.1016/j.semarthrit.2010.07.006. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

