ERK signaling is required for VEGF-A/VEGFR2-induced differentiation of porcine adipose-derived mesenchymal stem cells into endothelial cells
- PMID: 28499402
- PMCID: PMC5429549
- DOI: 10.1186/s13287-017-0568-4
ERK signaling is required for VEGF-A/VEGFR2-induced differentiation of porcine adipose-derived mesenchymal stem cells into endothelial cells
Abstract
Background: Cell-based therapy that can rejuvenate the endothelium with stimulated adipose-derived mesenchymal stem cells (AMSCs) is a promising therapeutic strategy for the re-endothelialization of denuded arteries at the stenting site. Previously, we have shown that silencing of MMP-2 and MMP-14 inhibits vascular endothelial growth factor receptor type 2 (VEGFR2) cleavage, and induces differentiation of AMSCs toward the endothelial cell (EC) lineage. In this study, we examined the underlying signaling pathways that regulate differentiation of AMSCs to ECs in vitro through VEGFR2.
Methods: AMSCs were isolated from porcine abdominal adipose tissue. The isolated AMSCs were characterized by positive expression of CD29, CD44, and CD90 and negative expression of CD11b and CD45. The isolated MSCs were transfected with siRNA to silence MMP-2, MMP-14, and angiotensin receptor 2 (ATR2). Cells were suspended either in endothelial basal media (EBM) or endothelial growth media (EGM) with various treatments. Flow cytometry was performed to examine the expression of EC markers, and western blot analysis was performed to examine the expression and activity of various kinases. Scratch assay was performed to examine the cell migration. Data were analyzed by ANOVA using PRISM GraphPad.
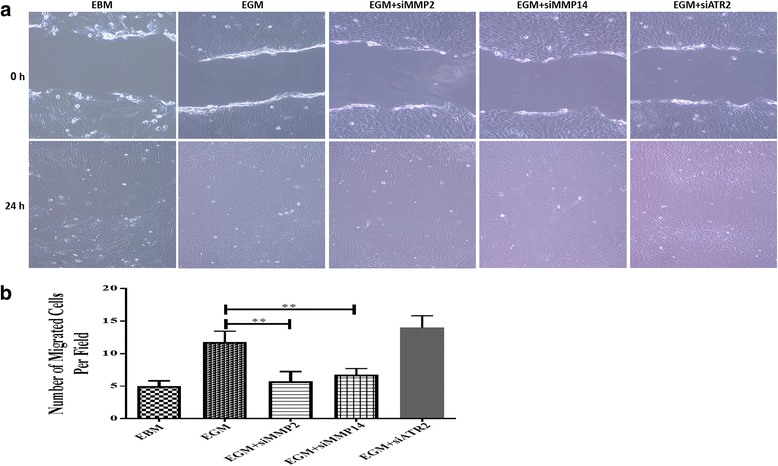
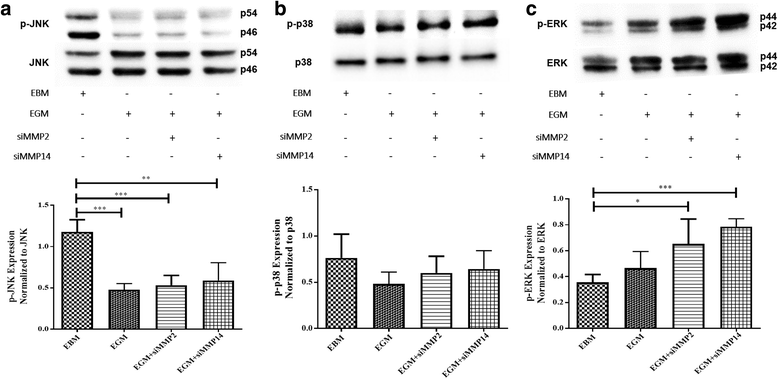
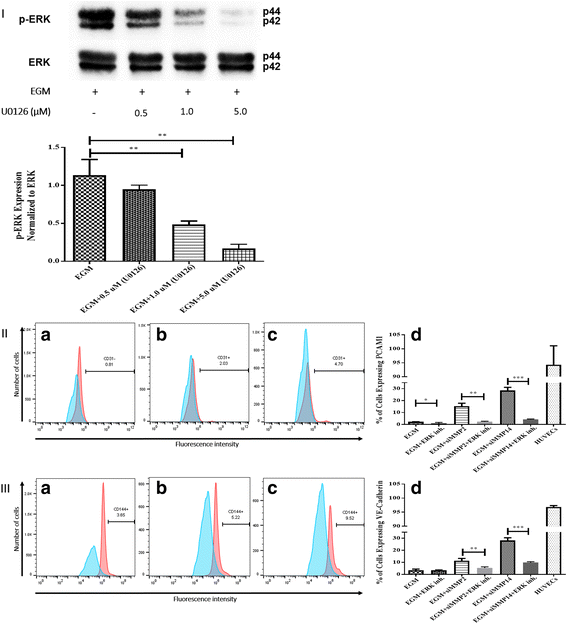
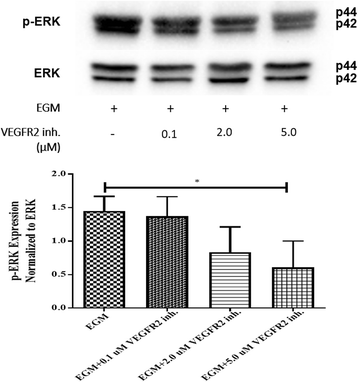
Results: After 10 days of stimulation for EC differentiation, the morphology of AMSCs changed to a morphology similar to that of ECs. Silencing MMP-2 and MMP-14 resulted in significant decrease in the number of migrated cells compared with the EGM-only group. ATR2 siRNA transfection did not affect the migration and differentiation of AMSCs to ECs. Stimulation of AMSCs for EC differentiation with or without MMP-2 or MMP-14 siRNA resulted in significant increase in p-ERK, and significant decrease in p-JNK. There was no significant change in p-p38 in all three groups compared with the EBM group. ERK inhibition resulted in significant decrease in the expression of EC markers in the EGM, EGM + MMP-2 siRNA, and EGM + MMP-14 siRNA groups. The VEGFR2 kinase inhibitor induced a dose-dependent inhibition of ERK.
Conclusion: The ERK signaling pathway is critical for VEGF-A/VEGFR2-induced differentiation of AMSCs into ECs. These findings provide new insights into the role of the ERK signaling pathway in AMSC differentiation to ECs for potential clinical use in cardiovascular diseases.
Keywords: Adipose-derived mesenchymal stem cells; Angiotensin type-2 receptor; Endothelial cell differentiation; Extracellular signal-regulated kinase 1/2; Mitogen-activated protein kinase; Stress activated protein kinase-2; Vascular endothelial growth factor receptor type 2; c-Jun NH2-terminal kinase.
Figures



Similar articles
-
MMP-2 and MMP-14 Silencing Inhibits VEGFR2 Cleavage and Induces the Differentiation of Porcine Adipose-Derived Mesenchymal Stem Cells to Endothelial Cells.Stem Cells Transl Med. 2017 May;6(5):1385-1398. doi: 10.1002/sctm.16-0329. Epub 2017 Feb 18. Stem Cells Transl Med. 2017. PMID: 28213979 Free PMC article.
-
VEGF and thrombin induce MKP-1 through distinct signaling pathways: role for MKP-1 in endothelial cell migration.Am J Physiol Cell Physiol. 2008 Jan;294(1):C241-50. doi: 10.1152/ajpcell.00187.2007. Epub 2007 Nov 14. Am J Physiol Cell Physiol. 2008. PMID: 18003751
-
Synergistic effect of angiotensin II on vascular endothelial growth factor-A-mediated differentiation of bone marrow-derived mesenchymal stem cells into endothelial cells.Stem Cell Res Ther. 2015 Jan 6;6(1):4. doi: 10.1186/scrt538. Stem Cell Res Ther. 2015. PMID: 25563650 Free PMC article.
-
Roles of cyclic adenosine monophosphate signaling in endothelial cell differentiation and arterial-venous specification during vascular development.Circ J. 2011;75(2):253-60. doi: 10.1253/circj.cj-10-0915. Epub 2010 Dec 20. Circ J. 2011. PMID: 21178292 Review.
-
Regulation of endothelial cell differentiation in embryonic vascular development and its therapeutic potential in cardiovascular diseases.Life Sci. 2021 Jul 1;276:119406. doi: 10.1016/j.lfs.2021.119406. Epub 2021 Mar 27. Life Sci. 2021. PMID: 33785330 Review.
Cited by
-
Chemical Distance Measurement and System Pharmacology Approach Uncover the Novel Protective Effects of Biotransformed Ginsenoside C-Mc against UVB-Irradiated Photoaging.Oxid Med Cell Longev. 2022 Feb 9;2022:4691576. doi: 10.1155/2022/4691576. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35186187 Free PMC article.
-
Extracellular matrix cues regulate the differentiation of pluripotent stem cell-derived endothelial cells.Front Cardiovasc Med. 2023 Jun 26;10:1169331. doi: 10.3389/fcvm.2023.1169331. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37435057 Free PMC article. Review.
-
Prognosis and Personalized Treatment Prediction in Different Mutation-Signature Hepatocellular Carcinoma.J Hepatocell Carcinoma. 2023 Feb 15;10:241-255. doi: 10.2147/JHC.S398431. eCollection 2023. J Hepatocell Carcinoma. 2023. PMID: 36815095 Free PMC article.
-
Bladder Cancer Cells Exert Pleiotropic Effects on Human Adipose-Derived Stem Cells.Life (Basel). 2022 Apr 7;12(4):549. doi: 10.3390/life12040549. Life (Basel). 2022. PMID: 35455040 Free PMC article.
-
Propofol attenuated TNF-α-modulated occludin expression by inhibiting Hif-1α/ VEGF/ VEGFR-2/ ERK signaling pathway in hCMEC/D3 cells.BMC Anesthesiol. 2019 Jul 9;19(1):127. doi: 10.1186/s12871-019-0788-5. BMC Anesthesiol. 2019. PMID: 31288745 Free PMC article.
References
-
- Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–95. - PubMed
-
- In ’t Anker PS, Noort WA, Scherjon SA, Kleijburg-Van der Keur C, Kruisselbrink AB, Van Bezooijen RL, et al. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica. 2003;88:845–52. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

