Anterograde monosynaptic transneuronal tracers derived from herpes simplex virus 1 strain H129
- PMID: 28499404
- PMCID: PMC5427628
- DOI: 10.1186/s13024-017-0179-7
Anterograde monosynaptic transneuronal tracers derived from herpes simplex virus 1 strain H129
Abstract
Background: Herpes simplex virus type 1 strain 129 (H129) has represented a promising anterograde neuronal circuit tracing tool, which complements the existing retrograde tracers. However, the current H129 derived tracers are multisynaptic, neither bright enough to label the details of neurons nor capable of determining direct projection targets as monosynaptic tracer.
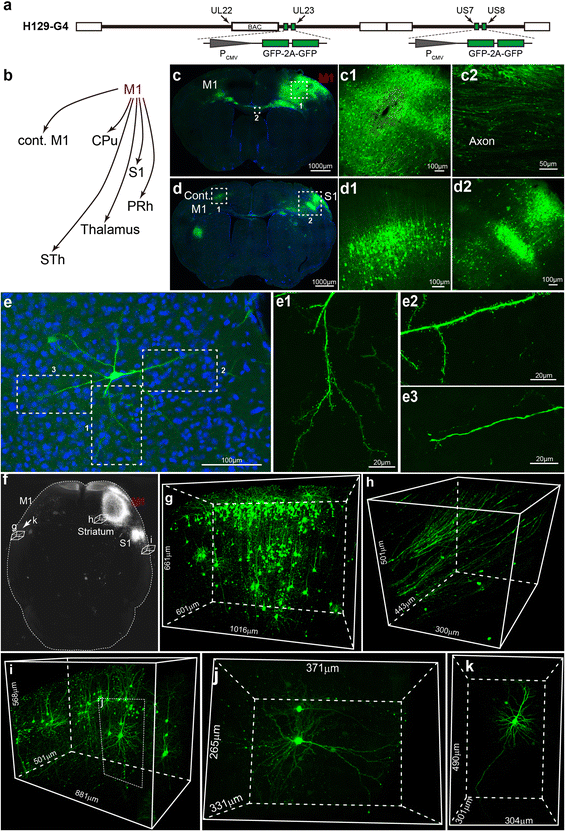
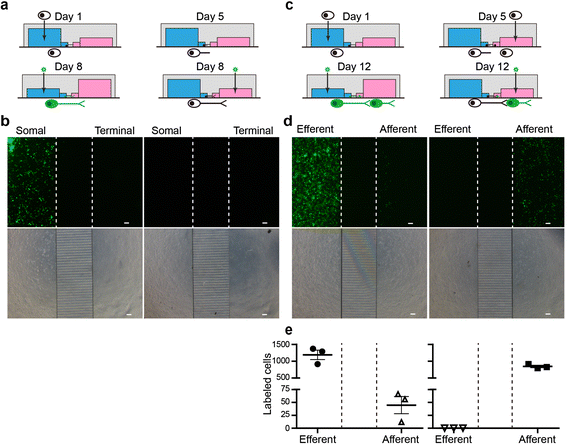
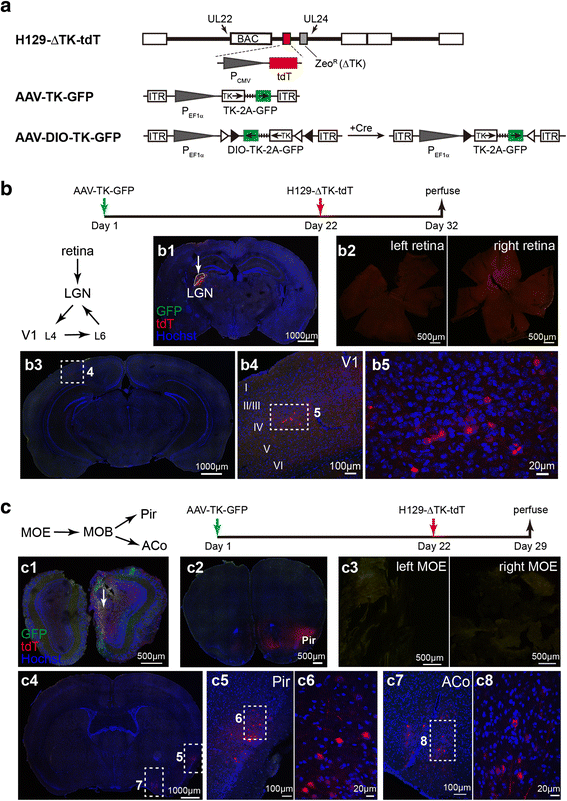
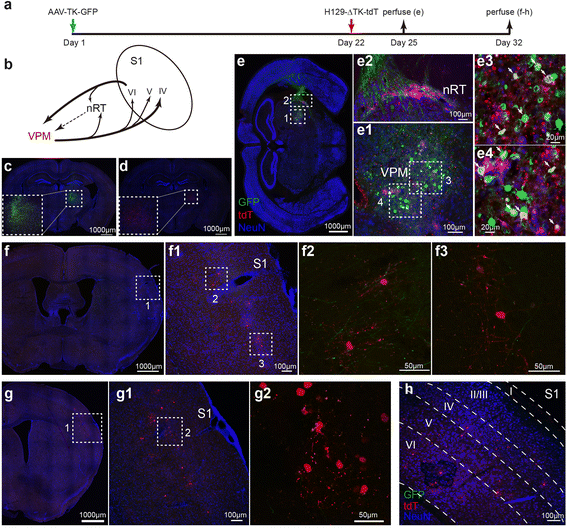
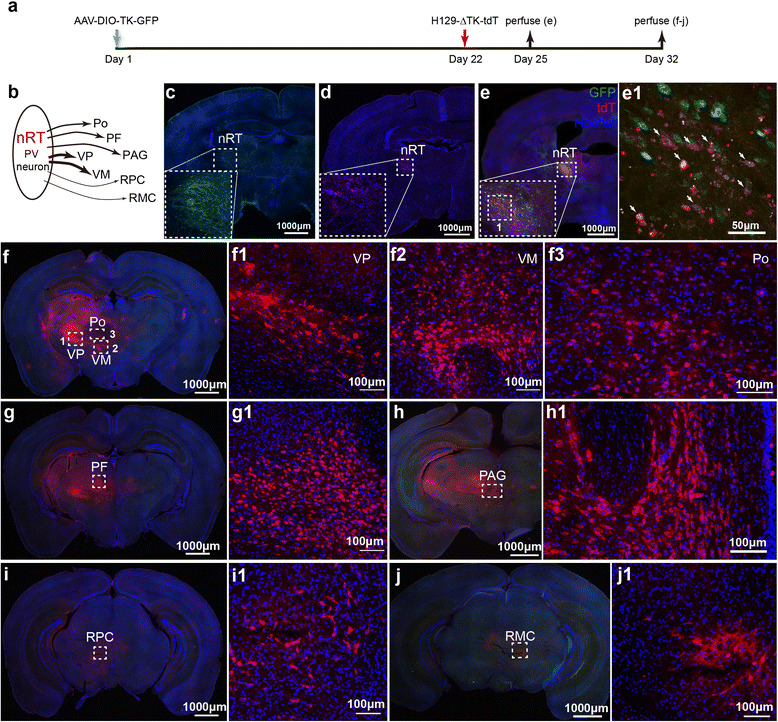
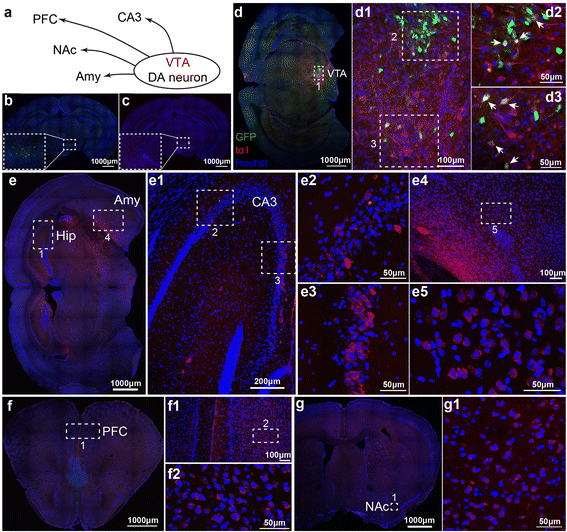
Methods: Based on the bacterial artificial chromosome of H129, we have generated a serial of recombinant viruses for neuronal circuit tracing. Among them, H129-G4 was obtained by inserting binary tandemly connected GFP cassettes into the H129 genome, and H129-ΔTK-tdT was obtained by deleting the thymidine kinase (TK) gene and adding tdTomato coding gene to the H129 genome. Then the obtained viral tracers were tested in vitro and in vivo for the tracing capacity.
Results: H129-G4 is capable of transmitting through multiple synapses, labeling the neurons by green florescent protein, and visualizing the morphological details of the labeled neurons. H129-ΔTK-tdT neither replicates nor spreads in neurons alone, but transmits to and labels the postsynaptic neurons with tdTomato in the presence of complementary expressed TK from a helper virus. H129-ΔTK-tdT is also capable to map the direct projectome of the specific neuron type in the given brain regions in Cre transgenic mice. In the tested brain regions where circuits are well known, the H129-ΔTK-tdT tracing patterns are consistent with the previous results.
Conclusions: With the assistance of the helper virus complimentarily expressing TK, H129-ΔTK-tdT replicates in the initially infected neuron, transmits anterogradely through one synapse, and labeled the postsynaptic neurons with tdTomato. The H129-ΔTK-tdT anterograde monosynaptic tracing system offers a useful tool for mapping the direct output in neuronal circuitry. H129-G4 is an anterograde multisynaptic tracer with a labeling signal strong enough to display the details of neuron morphology.
Keywords: Anterograde; H129; H129 strain; H129-G4; Herpes simplex virus type 1 (HSV-1); Monosynaptic; Multisynaptic; Neuronal tracer; tdT; ΔTK.
Figures
Similar articles
-
A novel H129-based anterograde monosynaptic tracer exhibits features of strong labeling intensity, high tracing efficiency, and reduced retrograde labeling.Mol Neurodegener. 2022 Jan 10;17(1):6. doi: 10.1186/s13024-021-00508-6. Mol Neurodegener. 2022. PMID: 35012591 Free PMC article.
-
Anterograde Viral Tracer Herpes Simplex Virus 1 Strain H129 Transports Primarily as Capsids in Cortical Neuron Axons.J Virol. 2020 Mar 31;94(8):e01957-19. doi: 10.1128/JVI.01957-19. Print 2020 Mar 31. J Virol. 2020. PMID: 31969440 Free PMC article.
-
HSV-1 H129-Derived Anterograde Neural Circuit Tracers: Improvements, Production, and Applications.Neurosci Bull. 2021 May;37(5):701-719. doi: 10.1007/s12264-020-00614-3. Epub 2020 Dec 24. Neurosci Bull. 2021. PMID: 33367996 Free PMC article.
-
Anterograde Neuronal Circuit Tracers Derived from Herpes Simplex Virus 1: Development, Application, and Perspectives.Int J Mol Sci. 2020 Aug 18;21(16):5937. doi: 10.3390/ijms21165937. Int J Mol Sci. 2020. PMID: 32824837 Free PMC article. Review.
-
Golgi staining-like retrograde labeling of brain circuits using rabies virus: Focus onto the striatonigral neurons.J Neurosci Methods. 2020 Oct 1;344:108872. doi: 10.1016/j.jneumeth.2020.108872. Epub 2020 Jul 18. J Neurosci Methods. 2020. PMID: 32693000 Review.
Cited by
-
A review of HSV pathogenesis, vaccine development, and advanced applications.Mol Biomed. 2024 Aug 29;5(1):35. doi: 10.1186/s43556-024-00199-7. Mol Biomed. 2024. PMID: 39207577 Free PMC article. Review.
-
Noncanonical projections to the hippocampal CA3 regulate spatial learning and memory by modulating the feedforward hippocampal trisynaptic pathway.PLoS Biol. 2021 Dec 20;19(12):e3001127. doi: 10.1371/journal.pbio.3001127. eCollection 2021 Dec. PLoS Biol. 2021. PMID: 34928938 Free PMC article.
-
USP49 negatively regulates cellular antiviral responses via deconjugating K63-linked ubiquitination of MITA.PLoS Pathog. 2019 Apr 3;15(4):e1007680. doi: 10.1371/journal.ppat.1007680. eCollection 2019 Apr. PLoS Pathog. 2019. PMID: 30943264 Free PMC article.
-
Differences in neurotropism and neurotoxicity among retrograde viral tracers.Mol Neurodegener. 2019 Feb 8;14(1):8. doi: 10.1186/s13024-019-0308-6. Mol Neurodegener. 2019. PMID: 30736827 Free PMC article.
-
Electroacupuncture Alleviates Motor Symptoms and Up-Regulates Vesicular Glutamatergic Transporter 1 Expression in the Subthalamic Nucleus in a Unilateral 6-Hydroxydopamine-Lesioned Hemi-Parkinsonian Rat Model.Neurosci Bull. 2018 Jun;34(3):476-484. doi: 10.1007/s12264-018-0213-y. Epub 2018 Mar 5. Neurosci Bull. 2018. PMID: 29508251 Free PMC article.
References
-
- McGovern AE, Driessen AK, Simmons DG, Powell J, Davis-Poynter N, Farrell MJ, Mazzone SB. Distinct brainstem and forebrain circuits receiving tracheal sensory neuron inputs revealed using a novel conditional anterograde transsynaptic viral tracing system. J Neurosci. 2015;35:7041–7055. doi: 10.1523/JNEUROSCI.5128-14.2015. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

