A feasibility randomised controlled trial of extended brief intervention for alcohol misuse in adults with mild to moderate intellectual disabilities living in the community; The EBI-LD study
- PMID: 28499440
- PMCID: PMC5427589
- DOI: 10.1186/s13063-017-1953-0
A feasibility randomised controlled trial of extended brief intervention for alcohol misuse in adults with mild to moderate intellectual disabilities living in the community; The EBI-LD study
Abstract
Background: Extended brief interventions (EBIs) are effective in targeting alcohol misuse in the general population. However, little is known of the effects of EBI in adults with intellectual (also known as learning) disabilities. In this feasibility trial we compared EBI with usual care for alcohol misuse in adults with mild to moderate Intellectual Disability (ID).
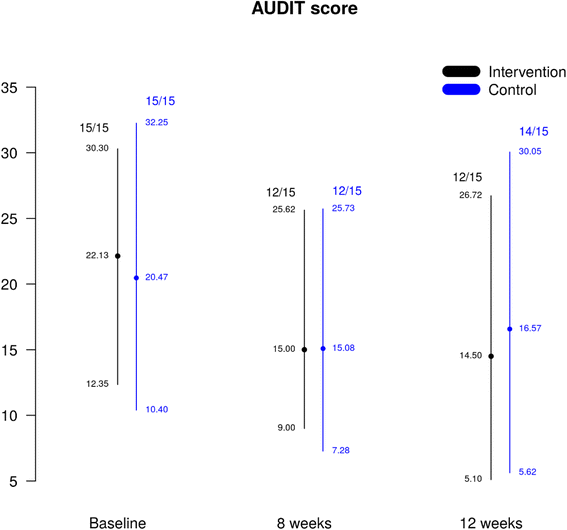
Methods: The study took place in three community ID networks of services in England. Participants aged 18-65 years with reported alcohol problems, a score ≥8 on the Alcohol Use Disorder Identification Test (AUDIT), and IQ <70 (+/5%CI) were recruited and were randomly allocated to either EBI (five weekly sessions and one follow-up at 8 weeks) and usual care or usual care alone. Research assistants were blind to arm allocation. Research assessments took place at baseline, 2 and 3 months. The primary outcome was reduction in alcohol consumption measured by the AUDIT. Preliminary health economic analysis was performed to investigate the costs of delivering EBI and the feasibility of a cost-effectiveness analysis in a full trial. The trial is closed.
Results: Participants were recruited from January 2014 to August 2015. Thirty individuals were randomised (15 in each arm) and provided primary outcome data. In regard to harmful drinking, at baseline, all the participants exceeded the relevant threshold. At 8 weeks, the proportion of participants with harmful drinking had decreased to 60% for both groups, and at 12 weeks it had decreased by 66°7% and 46°7% for the intervention and the control groups, respectively. The unit cost for the delivery of EBI is £430.
Conclusions: Recruitment to this trial has been proven challenging as prevalence of alcohol misuse in the targeted population was lower than anticipated. EBI may provide an effective low-intensity treatment for this population. Participants' and carers' feedback on their experience was overall positive. Further work needs to be undertaken to ascertain the group of participants that should be participating in a future definitive trial.
Trial registration: Psychological Intervention Alcohol Misuse Learning Disability; isrctn.com, identifier: ISRCTN58783633 . Registered on 17 December 2013.
Keywords: Alcohol misuse; Brief intervention; Intellectual disabilities.
Figures
Similar articles
-
Extended brief intervention to address alcohol misuse in people with mild to moderate intellectual disabilities living in the community (EBI-ID): study protocol for a randomised controlled trial.Trials. 2015 Mar 25;16:114. doi: 10.1186/s13063-015-0629-x. Trials. 2015. PMID: 25873255 Free PMC article. Clinical Trial.
-
Development and evaluation of a manual for extended brief intervention for alcohol misuse for adults with mild to moderate intellectual disabilities living in the community: The EBI-LD study manual.J Appl Res Intellect Disabil. 2017 Dec;30 Suppl 1:42-48. doi: 10.1111/jar.12409. Epub 2017 Sep 5. J Appl Res Intellect Disabil. 2017. PMID: 28875511 Clinical Trial.
-
Clinical and cost-effectiveness of an adapted intervention for preschoolers with moderate to severe intellectual disabilities displaying behaviours that challenge: the EPICC-ID RCT.Health Technol Assess. 2024 Jan;28(6):1-94. doi: 10.3310/JKTY6144. Health Technol Assess. 2024. PMID: 38329108 Free PMC article. Clinical Trial.
-
Screening and brief interventions for adolescent alcohol use disorders presenting through emergency departments: a research programme including two RCTs.Southampton (UK): NIHR Journals Library; 2020 Jan. Southampton (UK): NIHR Journals Library; 2020 Jan. PMID: 32011840 Free Books & Documents. Review.
-
Community Occupational Therapy in Dementia intervention for people with mild to moderate dementia and their family carers in the UK: the VALID research programme including RCT.Southampton (UK): National Institute for Health and Care Research; 2023 Jun. Southampton (UK): National Institute for Health and Care Research; 2023 Jun. PMID: 37463269 Free Books & Documents. Review.
Cited by
-
Benefits of temporary alcohol restriction: a feasibility randomized trial.Pilot Feasibility Stud. 2020 Jan 31;6:9. doi: 10.1186/s40814-020-0554-y. eCollection 2020. Pilot Feasibility Stud. 2020. PMID: 32021698 Free PMC article.
-
Toward Actionable Practice Parameters for "Dual Diagnosis": Principles of Assessment and Management for Co-Occurring Psychiatric and Intellectual/Developmental Disability.Curr Psychiatry Rep. 2020 Feb 1;22(2):9. doi: 10.1007/s11920-020-1127-8. Curr Psychiatry Rep. 2020. PMID: 32008108 Free PMC article. Review.
-
Understanding the effectiveness and underlying mechanisms of lifestyle modification interventions in adults with learning disabilities: a mixed-methods systematic review.Health Technol Assess. 2025 Feb;29(4):1-168. doi: 10.3310/BSTG4556. Health Technol Assess. 2025. PMID: 40025754 Free PMC article.
-
Interventions to prevent alcohol use: systematic review of economic evaluations.BJPsych Open. 2023 Jun 27;9(4):e117. doi: 10.1192/bjo.2023.81. BJPsych Open. 2023. PMID: 37365798 Free PMC article. Review.
-
Feasibility in Community Mental Health Research: A Scoping Review.Adm Policy Ment Health. 2025 Jul;52(4):800-818. doi: 10.1007/s10488-025-01457-8. Epub 2025 Jun 29. Adm Policy Ment Health. 2025. PMID: 40581893
References
-
- AAIDD . (American Association on Intellectual Developmental Disabilities) Intellectual disability: definition, classification, and systems of supports. Washington: AAIDD; 2010. - PubMed
-
- Hassiotis A, Strydom A, Hall I, et al. Psychiatric morbidity and social functioning among adults with borderline intelligence living in private households. J Intellect Disabil Res. 2008;52:95–106. - PubMed
-
- Lin E, Balogh R, McGarry C, Selick A, Dobranowski K, Wilton AS, Lunsky Y. Substance-related and addictive disorders among adults with intellectual and developmental disabilities (IDD): an Ontario population cohort study. BMJ Open. 2016;6(9):e011638. doi: 10.1136/bmjopen-2016-011638. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials