Intrabodies against the Polysialyltransferases ST8SiaII and ST8SiaIV inhibit Polysialylation of NCAM in rhabdomyosarcoma tumor cells
- PMID: 28499450
- PMCID: PMC5429572
- DOI: 10.1186/s12896-017-0360-7
Intrabodies against the Polysialyltransferases ST8SiaII and ST8SiaIV inhibit Polysialylation of NCAM in rhabdomyosarcoma tumor cells
Abstract
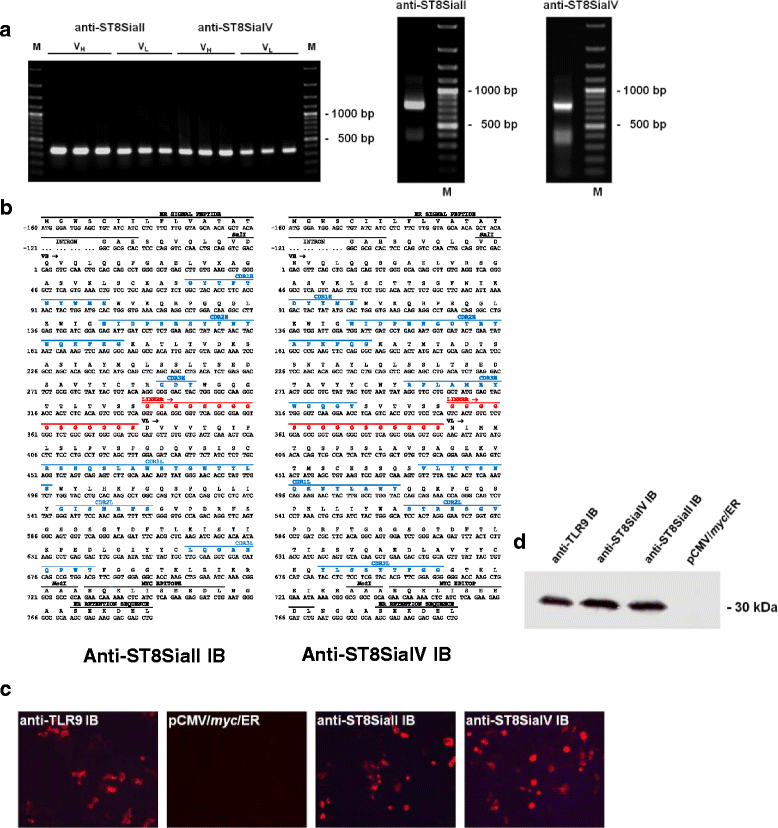
Background: Polysialic acid (polySia) is a carbohydrate modification of the neural cell adhesion molecule (NCAM), which is implicated in neural differentiation and plays an important role in tumor development and metastasis. Polysialylation of NCAM is mediated by two Golgi-resident polysialyltransferases (polyST) ST8SiaII and ST8SiaIV. Intracellular antibodies (intrabodies; IB) expressed inside the ER and retaining proteins passing the ER such as cell surface receptors or secretory proteins provide an efficient means of protein knockdown. To inhibit the function of ST8SiaII and ST8SiaIV specific ER IBs were generated starting from two corresponding hybridoma clones. Both IBs αST8SiaII-IB and αST8SiaIV-IB were constructed in the scFv format and their functions characterized in vitro and in vivo.
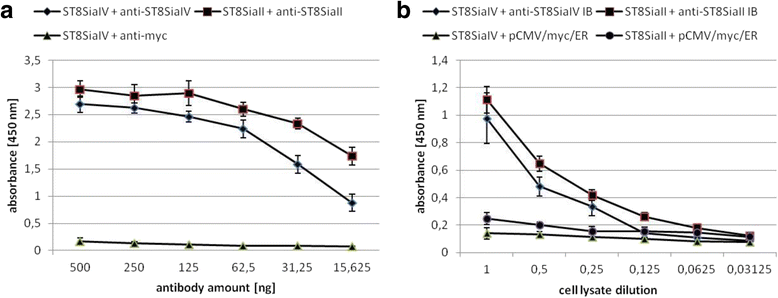
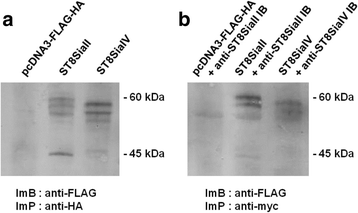
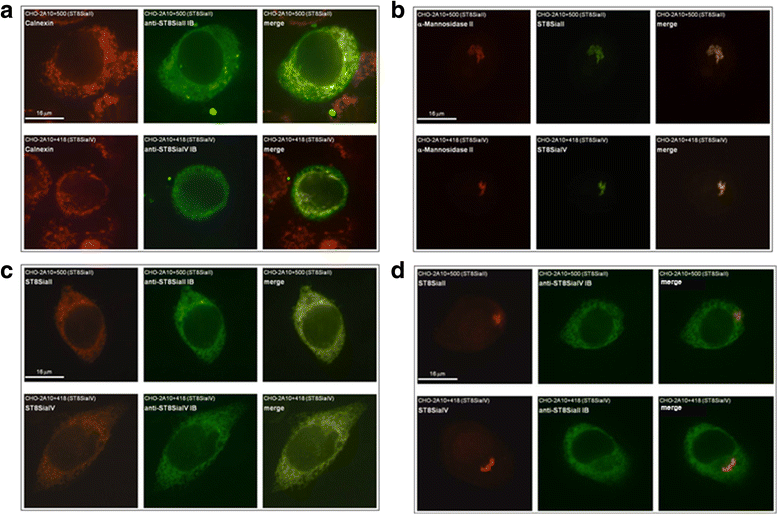
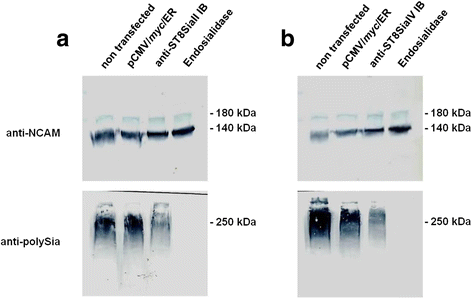
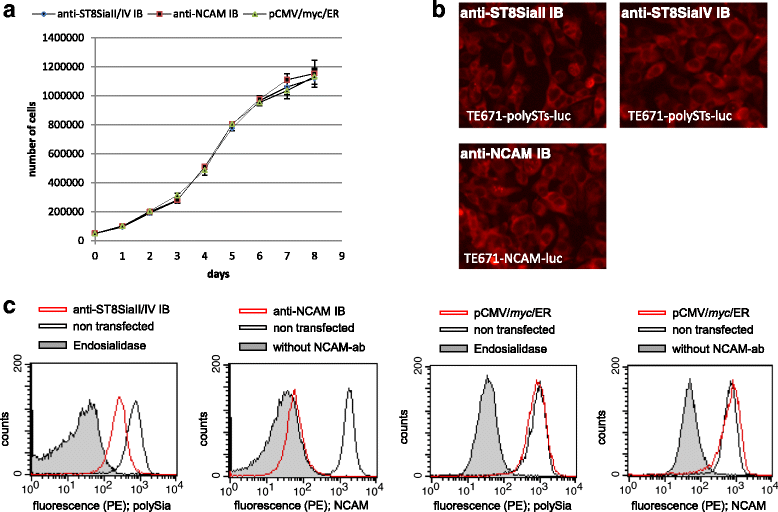
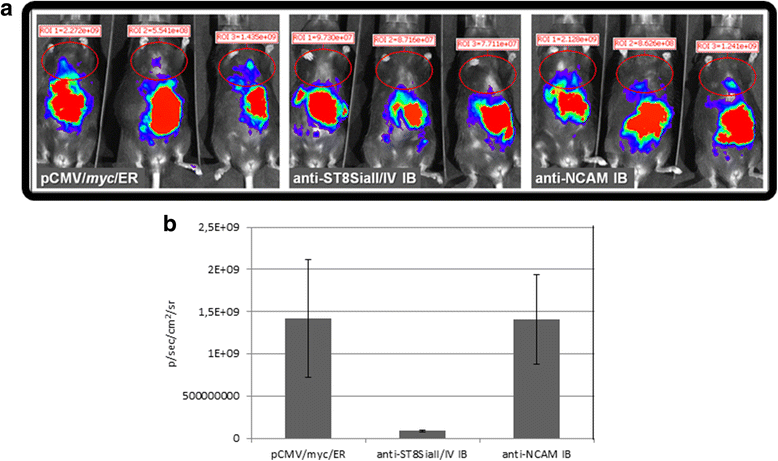
Results: IBs directed against the polySTs prevented the translocation of the enzymes from the ER to the Golgi-apparatus. Co-immunoprecipitation of ST8SiaII and ST8SiaIV with the corresponding IBs confirmed the intracellular interaction with their cognate antigens. In CHO cells overexpressing ST8SiaII and ST8SiaIV, respectively, the transfection with αST8SiaII-IB or αST8SiaIV-IB inhibited significantly the cell surface expression of polysialylated NCAM. Furthermore stable expression of ST8SiaII-IB, ST8SiaIV-IB and luciferase in the rhabdomyosarcoma cell line TE671 reduced cell surface expression of polySia and delayed tumor growth if cells were xenografted into C57BL/6 J RAG-2 mice.
Conclusion: Data obtained strongly indicate that αST8SiaII-IB and αST8SiaIV-IB are promising experimental tools to analyze the individual role of the two enzymes during brain development and during migration and proliferation of tumor cells.
Keywords: ER intrabodies; Neural cell adhesion molecule; Polysialic acid cell surface expression; Polysialyltransferases; Xenograft tumor mouse model.
Figures
Similar articles
-
Enzyme-dependent variations in the polysialylation of the neural cell adhesion molecule (NCAM) in vivo.J Biol Chem. 2008 Jan 4;283(1):17-28. doi: 10.1074/jbc.M707024200. Epub 2007 Nov 6. J Biol Chem. 2008. PMID: 17986444
-
Impact of the polysialyltransferases ST8SiaII and ST8SiaIV on polysialic acid synthesis during postnatal mouse brain development.J Biol Chem. 2008 Jan 18;283(3):1463-1471. doi: 10.1074/jbc.M708463200. Epub 2007 Nov 28. J Biol Chem. 2008. PMID: 18045870
-
Molecular defects that cause loss of polysialic acid in the complementation group 2A10.J Biol Chem. 2000 Oct 20;275(42):32861-70. doi: 10.1074/jbc.M003507200. J Biol Chem. 2000. PMID: 10921918
-
Polysialyltransferase: a new target in metastatic cancer.Curr Cancer Drug Targets. 2012 Oct;12(8):925-39. doi: 10.2174/156800912803251225. Curr Cancer Drug Targets. 2012. PMID: 22463390 Review.
-
Polysialylation of the neural cell adhesion molecule: interfering with polysialylation and migration in neuroblastoma cells.Arch Biochem Biophys. 2012 Aug 1;524(1):56-63. doi: 10.1016/j.abb.2012.04.011. Epub 2012 Apr 20. Arch Biochem Biophys. 2012. PMID: 22542522 Review.
Cited by
-
ER intrabody-mediated inhibition of interferon α secretion by mouse macrophages and dendritic cells.PLoS One. 2019 Apr 16;14(4):e0215062. doi: 10.1371/journal.pone.0215062. eCollection 2019. PLoS One. 2019. PMID: 30990863 Free PMC article.
-
Applying Antibodies Inside Cells: Principles and Recent Advances in Neurobiology, Virology and Oncology.BioDrugs. 2020 Aug;34(4):435-462. doi: 10.1007/s40259-020-00419-w. BioDrugs. 2020. PMID: 32301049 Free PMC article. Review.
-
Sialic acids as cellular markers of immunomodulatory action of dexamethasone on glioma cells of different immunogenicity.Mol Cell Biochem. 2019 May;455(1-2):147-157. doi: 10.1007/s11010-018-3478-6. Epub 2018 Nov 15. Mol Cell Biochem. 2019. PMID: 30443853 Free PMC article.
-
Glycolipids Recognized by A2B5 Antibody Promote Proliferation, Migration, and Clonogenicity in Glioblastoma Cells.Cancers (Basel). 2019 Aug 28;11(9):1267. doi: 10.3390/cancers11091267. Cancers (Basel). 2019. PMID: 31466399 Free PMC article.
-
TLR2 and TLR9 Blockade Using Specific Intrabodies Inhibits Inflammation-Mediated Pancreatic Cancer Cell Growth.Antibodies (Basel). 2024 Feb 1;13(1):11. doi: 10.3390/antib13010011. Antibodies (Basel). 2024. PMID: 38390872 Free PMC article.
References
-
- Rothbard JB, Brackenbury R, Cunningham BA, Edelman GM. Differences in the carbohydrate structures of neural cell-adhesion molecules from adult and embryonic chicken brains. J Biol Chem. 1982;257(18):11064–11069. - PubMed
-
- Finne J. Occurrence of unique polysialosyl carbohydrate units in glycoproteins of developing brain. J Biol Chem. 1982;257(20):11966–11970. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

