Cyclophosphamide and acrolein induced oxidative stress leading to deterioration of metaphase II mouse oocyte quality
- PMID: 28499912
- PMCID: PMC6854673
- DOI: 10.1016/j.freeradbiomed.2017.05.006
Cyclophosphamide and acrolein induced oxidative stress leading to deterioration of metaphase II mouse oocyte quality
Abstract
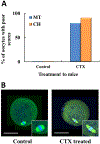
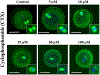
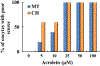
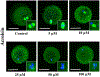
Cyclophosphamide (CTX) is a chemotherapeutic agent widely used to treat ovarian, breast, and hematological cancers as well as autoimmune disorders. Such chemotherapy is associated with reproductive failure and premature ovarian insufficiency. The mechanism by which CTX and/or its main metabolite, acrolein, affect female fertility remains unclear, but it is thought to be caused by an overproduction of reactive oxygen species (ROS). Here, we investigated the effect of CTX on metaphase II mouse oocytes obtained from treated animals (120mg/kg, 24h of single treatment), and oocytes directly exposed to increasing concentrations of CTX and acrolein (n=480; 0, 5, 10, 25, 50, and 100μM) with and without cumulus cells (CCs) for 45min which correlates to the time of maximum peak plasma concentrations after administration. Oocytes were fixed and subjected to indirect immunofluorescence and were scored based on microtubule spindle structure (MT) and chromosomal alignment (CH). Generation of ROS was evaluated using the Cellular Reactive Oxygen Species Detection Assay Kit. Deterioration of oocyte quality was noted when oocytes were obtained from CTX treated mice along with CTX and acrolein treated oocytes in a dose-dependent manner as shown by an increase in poor scores. Acrolein had an impact at a significantly lower level as compared to CTX, plateau at 10μM versus 50μM, respectively. These variation is are associated with the higher amount of ROS generated with acrolein exposure as compared to CTX (p<0.05). Utilization of antioxidant therapy and acrolein scavengers may mitigate the damaging effects of these compounds and help women undergoing such treatment.
Keywords: Acrolein; Chemotherapy; Chromosome alignment; Cyclophosphamide; Follicle dysfunction; Infertility; Meiotic spindle; Oocyte quality; Oxidative stress.
Copyright © 2017. Published by Elsevier Inc.
Figures



References
-
- Morgan S, et al., How do chemotherapeutic agents damage the ovary? Hum. Reprod. Update 18 (5) (2012) 525–535. - PubMed
-
- Blumenfeld Z, Chemotherapy and fertility, Best. Pract. Res. Clin. Obstet. Gynaecol 26 (3) (2012) 379–390. - PubMed
-
- Garrido-Colino C, et al., Current situation on fertility preservation in cancer patients in Spain: level of knowledge, information, and professional involvement, An. Pediatr (2016). - PubMed
-
- Nieman CL, et al., Cancer survivors and infertility: a review of a new problem and novel answers, J. Support Oncol 4 (4) (2006) 171–178. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

