The Upregulation of Integrin αDβ2 (CD11d/CD18) on Inflammatory Macrophages Promotes Macrophage Retention in Vascular Lesions and Development of Atherosclerosis
- PMID: 28500072
- PMCID: PMC5553324
- DOI: 10.4049/jimmunol.1602175
The Upregulation of Integrin αDβ2 (CD11d/CD18) on Inflammatory Macrophages Promotes Macrophage Retention in Vascular Lesions and Development of Atherosclerosis
Abstract
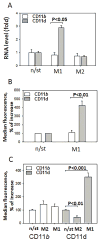
Macrophage accumulation is a critical step during development of chronic inflammation, initiating progression of many devastating diseases. Leukocyte-specific integrin αDβ2 (CD11d/CD18) is dramatically upregulated on macrophages at inflammatory sites. Previously we found that CD11d overexpression on cell surfaces inhibits in vitro cell migration due to excessive adhesion. In this study, we have investigated how inflammation-mediated CD11d upregulation contributes to macrophage retention at inflammatory sites during atherogenesis. Atherosclerosis was evaluated in CD11d-/-/ApoE-/- mice after 16 wk on a Western diet. CD11d deficiency led to a marked reduction in lipid deposition in aortas and isolated macrophages. Macrophage numbers in aortic sinuses of CD11d-/- mice were reduced without affecting their apoptosis and proliferation. Adoptive transfer of fluorescently labeled wild-type and CD11d-/- monocytes into ApoE-/- mice demonstrated similar recruitment from circulation, but reduced accumulation of CD11d-/- macrophages within the aortas. Furthermore, CD11d expression was significantly upregulated on macrophages in atherosclerotic lesions and M1 macrophages in vitro. Interestingly, expression of the related ligand-sharing integrin CD11b was not altered. This difference defines their distinct roles in the regulation of macrophage migration. CD11d-deficient M1 macrophages demonstrated improved migration in a three-dimensional fibrin matrix and during resolution of peritoneal inflammation, whereas migration of CD11b-/- M1 macrophages was not affected. These results prove the contribution of high densities of CD11d to macrophage arrest during atherogenesis. Because high expression of CD11d was detected in several inflammation-dependent diseases, we suggest that CD11d/CD18 upregulation on proinflammatory macrophages may represent a common mechanism for macrophage retention at inflammatory sites, thereby promoting chronic inflammation and disease development.
Copyright © 2017 by The American Association of Immunologists, Inc.
Figures





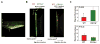

References
-
- Tobias P, Curtiss LK. Thematic review series: The immune system and atherogenesis. Paying the price for pathogen protection: toll receptors in atherogenesis. J Lipid Res. 2005;46:404–411. - PubMed
-
- Alexandraki K, Piperi C, Kalofoutis C, Singh J, Alaveras A, Kalofoutis A. Inflammatory process in type 2 diabetes: The role of cytokines. Ann N Y Acad Sci. 2006;1084:89–117. - PubMed
-
- Ouchi N, Kihara S, Funahashi T, Matsuzawa Y, Walsh K. Obesity, adiponectin and vascular inflammatory disease. Curr Opin Lipidol. 2003;14:561–566. - PubMed
-
- Subramanian S, Chait A. The effect of dietary cholesterol on macrophage accumulation in adipose tissue: implications for systemic inflammation and atherosclerosis. Curr Opin Lipidol. 2009;20:39–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

