Induced Pluripotent Stem Cell-Derived Cardiomyocytes Provide In Vivo Biological Pacemaker Function
- PMID: 28500172
- PMCID: PMC5434966
- DOI: 10.1161/CIRCEP.116.004508
Induced Pluripotent Stem Cell-Derived Cardiomyocytes Provide In Vivo Biological Pacemaker Function
Abstract
Background: Although multiple approaches have been used to create biological pacemakers in animal models, induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs) have not been investigated for this purpose. We now report pacemaker function of iPSC-CMs in a canine model.
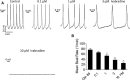
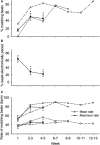
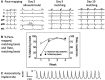
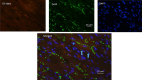
Methods and results: Embryoid bodies were derived from human keratinocytes, their action potential characteristics determined, and their gene expression profiles and markers of differentiation identified. Atrioventricular blocked dogs were immunosuppressed, instrumented with VVI pacemakers, and injected subepicardially into the anterobasal left ventricle with 40 to 75 rhythmically contracting embryoid bodies (totaling 1.3-2×106 cells). ECG and 24-hour Holter monitoring were performed biweekly. After 4 to 13 weeks, epinephrine (1 μg kg-1 min-1) was infused, and the heart removed for histological or electrophysiological study. iPSC-CMs largely lost the markers of pluripotency, became positive for cardiac-specific markers. and manifested If-dependent automaticity. Epicardial pacing of the injection site identified matching beats arising from that site by week 1 after implantation. By week 4, 20% of beats were electronically paced, 60% to 80% of beats were matching, and mean and maximal biological pacemaker rates were 45 and 75 beats per minute. Maximum night and day rates of matching beats were 53±6.9 and 69±10.4 beats per minute, respectively, at 4 weeks. Epinephrine increased rate of matching beats from 35±4.3 to 65±4.0 beats per minute. Incubation of embryoid bodies with the vital dye, Dil, revealed the persistence of injected cells at the site of administration.
Conclusions: iPSC-CMs can integrate into host myocardium and create a biological pacemaker. Although this is a promising development, rate and rhythm of the iPSC-CMs pacemakers remain to be optimized.
Keywords: action potentials; atrioventricular block; dogs; embryoid bodies; myocardium.
© 2017 The Authors.
Figures


Comment in
-
Induced Pluripotent Stem Cell-Based Treatment of Acquired Heart Block: The Battle for Tomorrow Has Begun!Circ Arrhythm Electrophysiol. 2017 May;10(5):e005331. doi: 10.1161/CIRCEP.117.005331. Circ Arrhythm Electrophysiol. 2017. PMID: 28500180 No abstract available.
References
-
- Rosen MR. Gene therapy and biological pacing. N Engl J Med. 2014;371:1158–1159. doi: 10.1056/NEJMcibr1408897. - PubMed
-
- Rosen MR, Robinson RB, Brink PR, Cohen IS. The road to biological pacing. Nat Rev Cardiol. 2011;8:656–666. doi: 10.1038/nrcardio.2011.120. - PubMed
-
- Plotnikov AN, Shlapakova I, Szabolcs MJ, Danilo P, Jr, Lorell BH, Potapova IA, Lu Z, Rosen AB, Mathias RT, Brink PR, Robinson RB, Cohen IS, Rosen MR. Xenografted adult human mesenchymal stem cells provide a platform for sustained biological pacemaker function in canine heart. Circulation. 2007;116:706–713. doi: 10.1161/CIRCULATIONAHA.107.703231. - PubMed
-
- Potapova I, Plotnikov A, Lu Z, Danilo P, Jr, Valiunas V, Qu J, Doronin S, Zuckerman J, Shlapakova IN, Gao J, Pan Z, Herron AJ, Robinson RB, Brink PR, Rosen MR, Cohen IS. Human mesenchymal stem cells as a gene delivery system to create cardiac pacemakers. Circ Res. 2004;94:952–959. doi: 10.1161/01.RES.0000123827.60210.72. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

