NKG2D Ligand-Targeted Bispecific T-Cell Engagers Lead to Robust Antitumor Activity against Diverse Human Tumors
- PMID: 28500232
- PMCID: PMC5531202
- DOI: 10.1158/1535-7163.MCT-16-0846
NKG2D Ligand-Targeted Bispecific T-Cell Engagers Lead to Robust Antitumor Activity against Diverse Human Tumors
Abstract
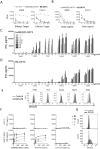
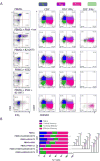
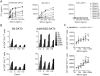
Two new bispecific T-cell engaging (BiTE) molecules with specificity for NKG2D ligands were developed and functionally characterized. One, huNKG2D-OKT3, was derived from the extracellular portion of the human NKG2D receptor fused to a CD3ε binding single-chain variable fragment (scFv), known as OKT3. NKG2D has multiple ligands, including MICA, which are expressed by a variety of malignant cells. A second molecule, B2-OKT3, was created in the tandem scFv BiTE format that targets MICA on tumor cells and CD3ε on human T cells. Both BiTEs specifically activated T cells to kill human tumor cell lines. Cytotoxicity by B2-OKT3, but not huNKG2D-OKT3, is blocked by soluble rMICA. The huNKG2D-OKT3 induced greater T-cell cytokine production in comparison with B2-OKT3. No T-cell pretreatment was required for IFNγ production upon coculture of B2-OKT3 or huNKG2D-OKT3 with T cells and target cells. The effector memory T-cell compartment was the primary source of IFNγ, and culture of T cells and these BiTEs with plate-bound rMICA showed ligand density-dependent production of IFNγ from both CD4+ and CD8+ T cells. There was 2-fold more IFNγ produced per CD8+ T cell and 5-fold greater percentage of CD8+ T cells producing IFNγ compared with CD4+ T cells. In addition, both BiTEs elicited significant antitumor responses against human metastatic melanoma tumor samples using autologous or healthy donor T cells. These data demonstrate the robust antitumor activity of these NKG2D ligand-binding bispecific proteins and support their further development for clinical use. Mol Cancer Ther; 16(7); 1335-46. ©2017 AACR.
©2017 American Association for Cancer Research.
Figures


Similar articles
-
Cancer immunotherapy using a bispecific NK receptor fusion protein that engages both T cells and tumor cells.Cancer Res. 2011 Mar 15;71(6):2066-76. doi: 10.1158/0008-5472.CAN-10-3200. Epub 2011 Jan 31. Cancer Res. 2011. PMID: 21282338 Free PMC article.
-
Bispecific killer cell engagers employing species cross-reactive NKG2D binders redirect human and murine lymphocytes to ErbB2/HER2-positive malignancies.Front Immunol. 2024 Aug 29;15:1457887. doi: 10.3389/fimmu.2024.1457887. eCollection 2024. Front Immunol. 2024. PMID: 39267747 Free PMC article.
-
Impaired tumor rejection by memory CD8 T cells in mice with NKG2D dysfunction.Int J Cancer. 2012 Oct 1;131(7):1601-10. doi: 10.1002/ijc.26191. Epub 2011 Aug 1. Int J Cancer. 2012. PMID: 21607945
-
TNK cells (NKG2D+ CD8+ or CD4+ T lymphocytes) in the control of human tumors.Cancer Immunol Immunother. 2009 May;58(5):801-8. doi: 10.1007/s00262-008-0635-x. Epub 2008 Dec 17. Cancer Immunol Immunother. 2009. PMID: 19089424 Free PMC article. Review.
-
Bispecific T-cell engagers for cancer immunotherapy.Immunol Cell Biol. 2015 Mar;93(3):290-6. doi: 10.1038/icb.2014.93. Epub 2014 Nov 4. Immunol Cell Biol. 2015. PMID: 25367186 Free PMC article. Review.
Cited by
-
Bispecific T cell engagers: an emerging therapy for management of hematologic malignancies.J Hematol Oncol. 2021 May 3;14(1):75. doi: 10.1186/s13045-021-01084-4. J Hematol Oncol. 2021. PMID: 33941237 Free PMC article. Review.
-
Toxicity Induced by a Bispecific T Cell-Redirecting Protein Is Mediated by Both T Cells and Myeloid Cells in Immunocompetent Mice.J Immunol. 2020 Jun 1;204(11):2973-2983. doi: 10.4049/jimmunol.1901401. Epub 2020 Apr 15. J Immunol. 2020. PMID: 32295875 Free PMC article.
-
Bispecific Antibodies Progression in Malignant Melanoma.Front Pharmacol. 2022 Mar 23;13:837889. doi: 10.3389/fphar.2022.837889. eCollection 2022. Front Pharmacol. 2022. PMID: 35401191 Free PMC article. Review.
-
Immune cell engagers in solid tumors: promises and challenges of the next generation immunotherapy.ESMO Open. 2021 Feb;6(1):100046. doi: 10.1016/j.esmoop.2020.100046. Epub 2021 Jan 25. ESMO Open. 2021. PMID: 33508733 Free PMC article. Review.
-
Bispecific NKG2D-CD3 and NKG2D-CD16 Fusion Proteins as Novel Treatment Option in Advanced Soft Tissue Sarcomas.Front Immunol. 2021 Apr 14;12:653081. doi: 10.3389/fimmu.2021.653081. eCollection 2021. Front Immunol. 2021. PMID: 33936075 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

