Fine-tune regulation of carboxypeptidase N1 controls vascular patterning during zebrafish development
- PMID: 28500283
- PMCID: PMC5431830
- DOI: 10.1038/s41598-017-01976-x
Fine-tune regulation of carboxypeptidase N1 controls vascular patterning during zebrafish development
Abstract
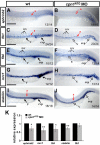
Vascular development is regulated by complicated signals and molecules in vertebrates. In this study, we characterized a novel function of carboxypeptidase N1 (Cpn1) in the vasculature. We show that cpn1 mRNA is expressed in developing vessels. The knockdown of cpn1 by morpholino injection impairs the growth of intersegmental vessels (ISV) and caudal vein plexus (CVP), suggesting the role of cpn1 in vascular development. We showed that vascular defects are not caused by cell death but are due to the impairment of migration and proliferation. Consistent with vascular growth defects, loss of cpn1 affects the expression of the vascular markers flt4, mrc1, flk, stabilin, and ephrinb2. Furthermore, the overexpression of cpn1 impaired the growth of ISV and CVP, but the remodeling expression of vascular markers was different from the knockdown of cpn1, indicating the differential regulation mechanisms in cpn1-overexpressing embryos. We examine the interaction between cpn1 and multiple signals and observed that cpn1 is regulated by Notch/VEGF signals for ISV growth and likely regulates BMP signals for CVP patterning. In conclusion, we demonstrate that cpn1 has a critical role in the vascular development of zebrafish. We also reveal a fine-tune regulation of cpn1 that controls vascular patterning mediated by multiple signals.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
-
- Childs S, Chen J-N, Garrity D, Fishman M. Patterning of angiogenesis in the zebrafish embryo. Development. 2002;129:973–982. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

