Labeling Extracellular Vesicles for Nanoscale Flow Cytometry
- PMID: 28500324
- PMCID: PMC5431945
- DOI: 10.1038/s41598-017-01731-2
Labeling Extracellular Vesicles for Nanoscale Flow Cytometry
Abstract
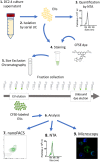
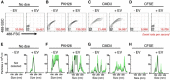
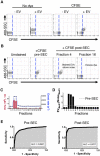
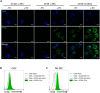
Extracellular vesicles (EVs), including exosomes and microvesicles, are 30-800 nm vesicles that are released by most cell types, as biological packages for intercellular communication. Their importance in cancer and inflammation makes EVs and their cargo promising biomarkers of disease and cell-free therapeutic agents. Emerging high-resolution cytometric methods have created a pressing need for efficient fluorescent labeling procedures to visualize and detect EVs. Suitable labels must be bright enough for one EV to be detected without the generation of label-associated artifacts. To identify a strategy that robustly labels individual EVs, we used nanoFACS, a high-resolution flow cytometric method that utilizes light scattering and fluorescence parameters along with sample enumeration, to evaluate various labels. Specifically, we compared lipid-, protein-, and RNA-based staining methods and developed a robust EV staining strategy, with the amine-reactive fluorescent label, 5-(and-6)-Carboxyfluorescein Diacetate Succinimidyl Ester, and size exclusion chromatography to remove unconjugated label. By combining nanoFACS measurements of light scattering and fluorescence, we evaluated the sensitivity and specificity of EV labeling assays in a manner that has not been described for other EV detection methods. Efficient characterization of EVs by nanoFACS paves the way towards further study of EVs and their roles in health and disease.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Imaging flow cytometry challenges the usefulness of classically used extracellular vesicle labeling dyes and qualifies the novel dye Exoria for the labeling of mesenchymal stromal cell-extracellular vesicle preparations.Cytotherapy. 2022 Jun;24(6):619-628. doi: 10.1016/j.jcyt.2022.02.003. Epub 2022 Mar 18. Cytotherapy. 2022. PMID: 35314115
-
Detection and Sorting of Extracellular Vesicles and Viruses Using nanoFACS.Curr Protoc Cytom. 2020 Dec;95(1):e81. doi: 10.1002/cpcy.81. Curr Protoc Cytom. 2020. PMID: 33332760 Free PMC article.
-
Flow cytometric analysis of extracellular vesicle subsets in plasma: impact of swarm by particles of non-interest.J Thromb Haemost. 2018 Jul;16(7):1423-1436. doi: 10.1111/jth.14154. Epub 2018 Jun 15. J Thromb Haemost. 2018. PMID: 29781099
-
The Methods of Choice for Extracellular Vesicles (EVs) Characterization.Int J Mol Sci. 2017 May 29;18(6):1153. doi: 10.3390/ijms18061153. Int J Mol Sci. 2017. PMID: 28555055 Free PMC article. Review.
-
Extracellular vesicles and their content in bioactive lipid mediators: more than a sack of microRNA.J Lipid Res. 2018 Nov;59(11):2037-2046. doi: 10.1194/jlr.R084640. Epub 2018 Apr 20. J Lipid Res. 2018. PMID: 29678959 Free PMC article. Review.
Cited by
-
Use of Nanosphere Self-Assembly to Pattern Nanoporous Membranes for the Study of Extracellular Vesicles.Nanoscale Adv. 2020 Oct;2(10):4427-4436. doi: 10.1039/D0NA00142B. Epub 2020 May 12. Nanoscale Adv. 2020. PMID: 33693309 Free PMC article.
-
Exosomes-Based Nanomedicine for Neurodegenerative Diseases: Current Insights and Future Challenges.Pharmaceutics. 2023 Jan 16;15(1):298. doi: 10.3390/pharmaceutics15010298. Pharmaceutics. 2023. PMID: 36678926 Free PMC article. Review.
-
Targeted Drug Delivery to the Central Nervous System Using Extracellular Vesicles.Pharmaceuticals (Basel). 2022 Mar 15;15(3):358. doi: 10.3390/ph15030358. Pharmaceuticals (Basel). 2022. PMID: 35337155 Free PMC article. Review.
-
Sperm acquire epididymis-derived proteins through epididymosomes.Hum Reprod. 2022 Apr 1;37(4):651-668. doi: 10.1093/humrep/deac015. Hum Reprod. 2022. PMID: 35137089 Free PMC article.
-
Blood Nanoparticles - Influence on Extracellular Vesicle Isolation and Characterization.Front Pharmacol. 2021 Nov 10;12:773844. doi: 10.3389/fphar.2021.773844. eCollection 2021. Front Pharmacol. 2021. PMID: 34867406 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

