Examining the efficacy of intravenous administration of predatory bacteria in rats
- PMID: 28500337
- PMCID: PMC5431856
- DOI: 10.1038/s41598-017-02041-3
Examining the efficacy of intravenous administration of predatory bacteria in rats
Abstract
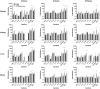
The proteobacteria Bdellovibrio bacteriovorus and Micavibrio aeruginosavorus are obligate predators of Gram-negative bacteria, and have been proposed to be used to treat multidrug-resistant bacterial infections. The ability of predatory bacteria to reduce bacterial burden in vivo within the lungs of rats has been demonstrated, but it was unknown if predatory bacteria can attenuate systemic bacterial burden administered intravenously. In this study, we first assessed the safety of intravenous inoculation of predatory bacteria in rats. No rat morbidity or adverse histopathology of various organs due to predatory bacteria administration was observed. An increase in proinflammatory cytokines (TNFα and KC/GRO) was observed at two hours post-inoculation; however, cytokines returned to baseline levels by 18 hours. Furthermore, bacterial dissemination analysis demonstrated that predatory bacteria were efficiently cleared from the host by 20 days post-injection. To determine whether predatory bacteria could reduce bacterial burden in vivo, Klebsiella pneumoniae was injected into the tail veins of rats and followed with multiple doses of predatory bacteria over 16 or 24 hours. Predatory bacteria were unable to significantly reduce K. pneumoniae burden in the blood or prevent dissemination to other organs. The results suggest that predatory bacteria may not be effective for treatment of acute blood infections.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




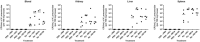
References
-
- Lambina VA, Afinogenova AV, Romay Penobad Z, Konovalova SM, Andreev LV. [New species of exoparasitic bacteria of the genus Micavibrio infecting gram-positive bacteria] Mikrobiologiia. 1983;52:777–780. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

