Retinoic acid regulates cell-shape and -death of E-FABP (FABP5)-immunoreactive septoclasts in the growth plate cartilage of mice
- PMID: 28500502
- PMCID: PMC5539264
- DOI: 10.1007/s00418-017-1578-0
Retinoic acid regulates cell-shape and -death of E-FABP (FABP5)-immunoreactive septoclasts in the growth plate cartilage of mice
Abstract
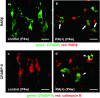
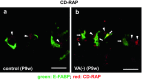
Septoclasts, which are mononuclear and spindle-shaped cells with many processes, have been considered to resorb the transverse septa of the growth plate (GP) cartilage at the chondro-osseous junction (COJ). We previously reported the expression of epidermal-type fatty acid-binding protein (E-FABP, FABP5) and localization of peroxisome proliferator-activated receptor (PPAR)β/δ, which mediates the cell survival or proliferation, in septoclasts. On the other hand, retinoic acid (RA) can bind to E-FABP and is stored abundantly in the GP cartilage. From these information, it is possible to hypothesize that RA in the GP is incorporated into septoclasts during the cartilage resorption and regulates the growth and/or death of septoclasts. To clarify the mechanism of the cartilage resorption induced by RA, we administered an overdose of RA or its precursor vitamin A (VA)-deficient diet to young mice. In mice of both RA excess and VA deficiency, septoclasts decreased in the number and cell size in association with shorter and lesser processes than those in normal mice, suggesting a substantial suppression of resorption by septoclasts in the GP cartilage. Lack of PPARβ/δ-expression, TUNEL reaction, RA receptor (RAR)β, and cellular retinoic acid-binding protein (CRABP)-II were induced in E-FABP-positive septoclasts under RA excess, suggesting the growth arrest/cell-death of septoclasts, whereas cartilage-derived retinoic acid-sensitive protein (CD-RAP) inducing the cell growth arrest or morphological changes was induced in septoclasts under VA deficiency. These results support and do not conflict with our hypothesis, suggesting that endogenous RA in the GP is possibly incorporated in septoclasts and utilized to regulate the activity of septoclasts resorbing the GP cartilage.
Keywords: E-FABP; Growth plate; Immunohistochemistry; Mouse; Retinoic acid; Septoclast.
Conflict of interest statement
Conflict of interest
The authors declare that they have no conflict of interest.
Statement on the welfare of animals
All animal procedures were in accordance with the Guidelines for care and use of Laboratory Animals of Meikai University School of Dentistry, and these experiments were approved by Meikai University Animal Ethics Committee (A1602).
Funding
This work was supported by JSPS KAKENHI Grant Numbers JP16K11457 to Y. B. and JP26462796 to O. A. and Meikai University Miyata Research Grant (2016 A to Y. B.).
Figures







References
-
- Blesch A, Bosserhoff AK, Apfel R, Behl C, Hessdoerfer B, Schmitt A, Jachimczak P, Lottspeich F, Buettner R, Bogdahn U. Cloning of a novel malignant melanoma-derived growth-regulatory protein, MIA. Cancer Res. 1994;54:5695–5701. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

