Expression and localization of myosin VI in developing mouse spermatids
- PMID: 28500503
- PMCID: PMC5602056
- DOI: 10.1007/s00418-017-1579-z
Expression and localization of myosin VI in developing mouse spermatids
Abstract
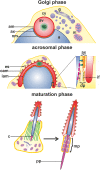
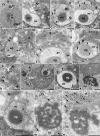
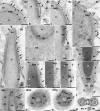
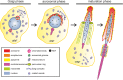
Myosin VI (MVI) is a versatile actin-based motor protein that has been implicated in a variety of different cellular processes, including endo- and exocytic vesicle trafficking, Golgi morphology, and actin structure stabilization. A role for MVI in crucial actin-based processes involved in sperm maturation was demonstrated in Drosophila. Because of the prominence and importance of actin structures in mammalian spermiogenesis, we investigated whether MVI was associated with actin-mediated maturation events in mammals. Both immunofluorescence and ultrastructural analyses using immunogold labeling showed that MVI was strongly linked with key structures involved in sperm development and maturation. During the early stage of spermiogenesis, MVI is associated with the Golgi and with coated and uncoated vesicles, which fuse to form the acrosome. Later, as the acrosome spreads to form a cap covering the sperm nucleus, MVI is localized to the acroplaxome, an actin-rich structure that anchors the acrosome to the nucleus. Finally, during the elongation/maturation phase, MVI is associated with the actin-rich structures involved in nuclear shaping: the acroplaxome, manchette, and Sertoli cell actin hoops. Since this is the first report of MVI expression and localization during mouse spermiogenesis and MVI partners in developing sperm have not yet been identified, we discuss some probable roles for MVI in this process. During early stages, MVI is hypothesized to play a role in Golgi morphology and function as well as in actin dynamics regulation important for attachment of developing acrosome to the nuclear envelope. Next, the protein might also play anchoring roles to help generate forces needed for spermatid head elongation. Moreover, association of MVI with actin that accumulates in the Sertoli cell ectoplasmic specialization and other actin structures in surrounding cells suggests additional MVI functions in spermatid movement across the seminiferous epithelium and in sperm release.
Keywords: Actin; Immunocytochemistry; Myosin VI splice variants; Spermiogenesis; Ultrastructure.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures





Similar articles
-
Loss of myosin VI expression affects acrosome/acroplaxome complex morphology during mouse spermiogenesis†.Biol Reprod. 2020 Aug 21;103(3):521-533. doi: 10.1093/biolre/ioaa071. Biol Reprod. 2020. PMID: 32412041 Free PMC article.
-
The actin-based motor myosin Va is a component of the acroplaxome, an acrosome-nuclear envelope junctional plate, and of manchette-associated vesicles.Cytogenet Genome Res. 2003;103(3-4):337-44. doi: 10.1159/000076822. Cytogenet Genome Res. 2003. PMID: 15051957
-
The acroplaxome is the docking site of Golgi-derived myosin Va/Rab27a/b- containing proacrosomal vesicles in wild-type and Hrb mutant mouse spermatids.Biol Reprod. 2004 May;70(5):1400-10. doi: 10.1095/biolreprod.103.025346. Epub 2004 Jan 14. Biol Reprod. 2004. PMID: 14724135
-
Molecular biology of sperm head shaping.Soc Reprod Fertil Suppl. 2007;65:33-43. Soc Reprod Fertil Suppl. 2007. PMID: 17644953 Review.
-
The acrosome-acroplaxome-manchette complex and the shaping of the spermatid head.Arch Histol Cytol. 2004 Nov;67(4):271-84. doi: 10.1679/aohc.67.271. Arch Histol Cytol. 2004. PMID: 15700535 Review.
Cited by
-
Loss of myosin VI expression affects acrosome/acroplaxome complex morphology during mouse spermiogenesis†.Biol Reprod. 2020 Aug 21;103(3):521-533. doi: 10.1093/biolre/ioaa071. Biol Reprod. 2020. PMID: 32412041 Free PMC article.
-
Myosin VI Is Associated With the Endoplasmic Reticulum in Regions of Sertoli Cells Containing Tubulobulbar Complexes.Cytoskeleton (Hoboken). 2025 Jun;82(6):333-343. doi: 10.1002/cm.21949. Epub 2024 Oct 10. Cytoskeleton (Hoboken). 2025. PMID: 39390674 Free PMC article.
-
In focus in HCB.Histochem Cell Biol. 2019 Dec;152(6):391-395. doi: 10.1007/s00418-019-01831-2. Histochem Cell Biol. 2019. PMID: 31760465 No abstract available.
-
Testis-specific serine kinase 3 is required for sperm morphogenesis and male fertility.Andrology. 2023 Jul;11(5):826-839. doi: 10.1111/andr.13314. Epub 2022 Nov 16. Andrology. 2023. PMID: 36306217 Free PMC article.
-
Knockout of serine-rich single-pass membrane protein 1 (Ssmem1) causes globozoospermia and sterility in male mice†.Biol Reprod. 2020 Aug 4;103(2):244-253. doi: 10.1093/biolre/ioaa040. Biol Reprod. 2020. PMID: 32301969 Free PMC article.
References
-
- Behnen M, Murk K, Kursula P, Cappallo-Obermann H, Rothkegel M, Kirszenbaum AL, Kirchhoff C. Testis-expressed profilins 3 and 4 show distinct functional characteristics and localize in the acroplaxome–manchette complex in spermatids. BMC Cell Biol. 2009;10:34. doi: 10.1186/1471-2121-10-34. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

