Optimizing the dose in cancer patients treated with imatinib, sunitinib and pazopanib
- PMID: 28500677
- PMCID: PMC5595974
- DOI: 10.1111/bcp.13327
Optimizing the dose in cancer patients treated with imatinib, sunitinib and pazopanib
Abstract
Aim: Fixed dose oral tyrosine kinase inhibitors imatinib, sunitinib and pazopanib show a high interpatient variability in plasma exposure. A relationship between plasma exposure and treatment outcome has been established, which supports the rationale for dose optimization of these drugs. The aim of this study was to monitor how many patients reached adequate trough levels after therapeutic drug monitoring-based dose optimization in daily practice.
Methods: A cohort study was performed in patients treated with imatinib, sunitinib or pazopanib of whom follow-up drug levels were measured between August 2012 and April 2016. Patients' characteristics were collected by reviewing electronic patient records. Drug levels were measured using high-performance liquid chromatography coupled with tandem mass spectrometry and trough levels were estimated using a predefined algorithm. Dose interventions were proposed based on trough levels.
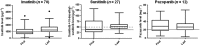
Results: In total, 396 trough levels were determined in 109 patients. Median sample frequency per patient was 3. During the first measurement only 38% of patients showed trough levels within the predefined target ranges despite standard dosing; 52% of the patients showed drug levels below and 10% above the target range. In 35 out of 41 patients (85%) dose interventions led to adequate trough levels. Eventually, 64% of the total cohort reached adequate trough levels.
Conclusions: Dose optimization proved an effective tool to reach adequate trough levels in patients treated with imatinib, sunitinib and pazopanib. The percentage of patients with adequate trough levels increased from 38 to 64%. Therapeutic drug monitoring may add to the improvement of efficacy and reduction of toxicity and costs of these treatments.
Keywords: imatinib; individualized dosing; pazopanib; pharmacokinetics; sunitinib; therapeutic drug monitoring.
© 2017 The British Pharmacological Society.
Figures
References
-
- de Wit D, Guchelaar HJ, den Hartigh J, Gelderblom H, van Erp NP. Individualized dosing of tyrosine kinase inhibitors: are we there yet? Drug Discov Today 2015; 20: 18–36. - PubMed
-
- Lankheet NA, Knapen LM, Schellens JH, Beijnen JH, Steeghs N, Huitema AD. Plasma concentrations of tyrosine kinase inhibitors imatinib, erlotinib, and sunitinib in routine clinical outpatient cancer care. Ther Drug Monit 2014; 36: 326–334. - PubMed
-
- Eechoute K, Fransson MN, Reyners AK, de Jong FA, Sparreboom A, van der Graaf WT, et al. A long‐term prospective population pharmacokinetic study on imatinib plasma concentrations in GIST patients. Clin Cancer Res 2012; 18: 5780–5787. - PubMed
-
- Klumpen HJ, Samer CF, Mathijssen RH, Schellens JH, Gurney H. Moving towards dose individualization of tyrosine kinase inhibitors. Cancer Treat Rev 2011; 37: 251–260. - PubMed
-
- Yu H, van Erp N, Bins S, Mathijssen RH, Schellens JH, Beijnen JH, et al. Development of a pharmacokinetic model to describe the complex pharmacokinetics of Pazopanib in cancer patients. Clin Pharmacokinet 2017; 56: 293–303. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

