Interventions for preventing silent cerebral infarcts in people with sickle cell disease
- PMID: 28500860
- PMCID: PMC5460750
- DOI: 10.1002/14651858.CD012389.pub2
Interventions for preventing silent cerebral infarcts in people with sickle cell disease
Update in
-
Interventions for preventing silent cerebral infarcts in people with sickle cell disease.Cochrane Database Syst Rev. 2020 Apr 6;4(4):CD012389. doi: 10.1002/14651858.CD012389.pub3. Cochrane Database Syst Rev. 2020. PMID: 32250453 Free PMC article.
Abstract
Background: Sickle cell disease (SCD) is one of the commonest severe monogenic disorders in the world, due to the inheritance of two abnormal haemoglobin (beta globin) genes. SCD can cause severe pain, significant end-organ damage, pulmonary complications, and premature death. Silent cerebral infarcts are the commonest neurological complication in children and probably adults with SCD. Silent cerebral infarcts also affect academic performance, increase cognitive deficits and may lower intelligence quotient.
Objectives: To assess the effectiveness of interventions to reduce or prevent silent cerebral infarcts in people with SCD.
Search methods: We searched for relevant trials in the Cochrane Library, MEDLINE (from 1946), Embase (from 1974), the Transfusion Evidence Library (from 1980), and ongoing trial databases; all searches current to 19 September 2016. We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group Trials Register: 06 October 2016.
Selection criteria: Randomised controlled trials comparing interventions to prevent silent cerebral infarcts in people with SCD. There were no restrictions by outcomes examined, language or publication status.
Data collection and analysis: We used standard Cochrane methodological procedures.
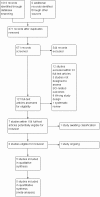
Main results: We included five trials (660 children or adolescents) published between 1998 and 2016. Four of the five trials were terminated early. The vast majority of participants had the haemoglobin (Hb)SS form of SCD. One trial focused on preventing silent cerebral infarcts or stroke; three trials were for primary stroke prevention and one trial dealt with secondary stroke prevention.Three trials compared the use of regular long-term red blood cell transfusions to standard care. Two of these trials included children with no previous long-term transfusions: one in children with normal transcranial doppler (TCD) velocities; and one in children with abnormal TCD velocities. The third trial included children and adolescents on long-term transfusion.Two trials compared the drug hydroxyurea and phlebotomy to long-term transfusions and iron chelation therapy: one in primary prevention (children), and one in secondary prevention (children and adolescents).The quality of the evidence was moderate to very low across different outcomes according to GRADE methodology. This was due to trials being at high risk of bias because they were unblinded; indirectness (available evidence was only for children with HbSS); and imprecise outcome estimates. Long-term red blood cell transfusions versus standard care Children with no previous long-term transfusions and higher risk of stroke (abnormal TCD velocities or previous history of silent cerebral infarcts) Long-term red blood cell transfusions may reduce the incidence of silent cerebral infarcts in children with abnormal TCD velocities, risk ratio (RR) 0.11 (95% confidence interval (CI) 0.02 to 0.86) (one trial, 124 participants, low-quality evidence); but make little or no difference to the incidence of silent cerebral infarcts in children with previous silent cerebral infarcts on magnetic resonance imaging and normal or conditional TCDs, RR 0.70 (95% CI 0.23 to 2.13) (one trial, 196 participants, low-quality evidence).No deaths were reported in either trial.Long-term red blood cell transfusions may reduce the incidence of: acute chest syndrome, RR 0.24 (95% CI 0.12 to 0.49) (two trials, 326 participants, low-quality evidence); and painful crisis, RR 0.63 (95% CI 0.42 to 0.95) (two trials, 326 participants, low-quality evidence); and probably reduces the incidence of clinical stroke, RR 0.12 (95% CI 0.03 to 0.49) (two trials, 326 participants, moderate-quality evidence).Long-term red blood cell transfusions may improve quality of life in children with previous silent cerebral infarcts (difference estimate -0.54; 95% confidence interval -0.92 to -0.17; one trial; 166 participants), but may have no effect on cognitive function (least squares means: 1.7, 95% CI -1.1 to 4.4) (one trial, 166 participants, low-quality evidence). Transfusions continued versus transfusions halted: children and adolescents with normalised TCD velocities (79 participants; one trial)Continuing red blood cell transfusions may reduce the incidence of silent cerebral infarcts, RR 0.29 (95% CI 0.09 to 0.97 (low-quality evidence).We are very uncertain whether continuing red blood cell transfusions has any effect on all-cause mortality, Peto odds ratio (OR) 8.00 (95% CI 0.16 to 404.12); or clinical stroke, RR 0.22 (95% CI 0.01 to 4.35) (very low-quality evidence).The trial did not report: comparative numbers for SCD-related adverse events; quality of life; or cognitive function. Hydroxyurea and phlebotomy versus transfusions and chelation Primary prevention, children (121 participants; one trial)We are very uncertain whether switching to hydroxyurea and phlebotomy has any effect on: silent cerebral infarcts (no infarcts); all-cause mortality (no deaths); risk of stroke (no strokes); or SCD-related complications, RR 1.52 (95% CI 0.58 to 4.02) (very low-quality evidence). Secondary prevention, children and adolescents with a history of stroke (133 participants; one trial)We are very uncertain whether switching to hydroxyurea and phlebotomy has any effect on: silent cerebral infarcts, Peto OR 7.28 (95% CI 0.14 to 366.91); all-cause mortality, Peto OR 1.02 (95%CI 0.06 to 16.41); or clinical stroke, RR 14.78 (95% CI 0.86 to 253.66) (very low-quality evidence).Switching to hydroxyurea and phlebotomy may increase the risk of SCD-related complications, RR 3.10 (95% CI 1.42 to 6.75) (low-quality evidence).Neither trial reported on quality of life or cognitive function.
Authors' conclusions: We identified no trials for preventing silent cerebral infarcts in adults, or in children who do not have HbSS SCD.Long-term red blood cell transfusions may reduce the incidence of silent cerebral infarcts in children with abnormal TCD velocities, but may have little or no effect on children with normal TCD velocities. In children who are at higher risk of stroke and have not had previous long-term transfusions, long-term red blood cell transfusions probably reduce the risk of stroke, and other SCD-related complications (acute chest syndrome and painful crises).In children and adolescents at high risk of stroke whose TCD velocities have normalised, continuing red blood cell transfusions may reduce the risk of silent cerebral infarcts. No treatment duration threshold has been established for stopping transfusions.Switching to hydroxyurea with phlebotomy may increase the risk of silent cerebral infarcts and SCD-related serious adverse events in secondary stroke prevention.All other evidence in this review is of very low-quality.
Conflict of interest statement
Lise Estcourt: partly funded by the NIHR Cochrane Programme Grant ‐ Safe and Appropriate Use of Blood Components.
Patricia Fortin: funded by the NIHR Cochrane Programme Grant ‐ Safe and Appropriate Use of Blood Components.
Sally Hopewell: partly funded by the NIHR Cochrane Programme Grant ‐ Safe and Appropriate Use of Blood Components.
Marialena Trivella: partly funded by the NIHR Cochrane Programme Grant ‐ Safe and Appropriate Use of Blood Components.
Miguel Abboud: has received research funding from Mast Therapeutics, Eli Lilly, Shire, Dilaforette, he also serves on advisory boards and steering committees for Eli Lilly, Pfizer, Astra Zeneca and Novo. These activities had no relationship to the management of neurologic complications in sickle cell disease.
Figures







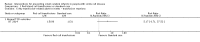


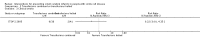





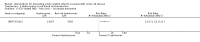






Update of
-
Interventions for preventing silent cerebral infarcts in people with sickle cell disease.Cochrane Database Syst Rev. 2016 Oct;2016(10):CD012389. doi: 10.1002/14651858.CD012389. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2017 May 13;5:CD012389. doi: 10.1002/14651858.CD012389.pub2. PMID: 28344510 Free PMC article. Updated.
Comment in
-
Are current interventions for preventing silent cerebral infarcts in people with sickle cell disease effective and safe? A Cochrane Review summary with commentary.Dev Med Child Neurol. 2021 Apr;63(4):372-373. doi: 10.1111/dmcn.14826. Epub 2021 Feb 2. Dev Med Child Neurol. 2021. PMID: 33533031 No abstract available.
References
References to studies included in this review
-
- Beverung LM, Strouse JJ, Hulbert ML, Neville K, Liem RI, Inusa B, et al. Health‐related quality of life in children with sickle cell anemia: impact of blood transfusion therapy. American Journal of Hematology 2015;90(2):139‐43. - PMC - PubMed
- Bhatnagar P, Casella EB, Arking DE, Casella JF. Genome‐wide association for silent cerebral infarction (SCI) in sickle cell disease: the silent infarct transfusion trial (SIT) cohort. Blood 2009;114(22):2563. - PubMed
- Bhatnagar P, Purvis S, Barron‐Casella E, DeBaun MR, Casella JF, Arking DE, et al. Genome‐wide association study identifies genetic variants influencing F‐cell levels in sickle‐cell patients. Journal of Human Genetics 2011;56(4):316‐23. - PMC - PubMed
- Bhatnagar P, Purvis S, Barron‐Casella E, DeBaun MR, Casella JF, Arking DE, et al. Genome‐wide association study identifies genetic variants influencing F‐cell levels in sickle‐cell patients. Journal of Human Genetics 2011;56(4):316‐23. Supplementary information. http://www.nature.com/jhg/journal/v56/n4/suppinfo/jhg201112s1.html. - PMC - PubMed
- Casella JF, King AA, Barton B, White DA, Noetzel MJ, Ichord RN, et al. Design of the silent cerebral infarct transfusion (SIT) trial. Pediatric Hematology & Oncology 2010;27(2):69‐89. - PMC - PubMed
- DeBaun MR, Gordon M, McKinstry RC, Noetzel MJ, White DA, Sarnaik SA, et al. Controlled trial of transfusions for silent cerebral infarcts in sickle cell anemia. New England Journal of Medicine 2014;371(8):699‐710. - PMC - PubMed
- DeBaun MR, Sarnaik SA, Rodeghier MJ, Minniti CP, Howard TH, Iyer RV, et al. Associated risk factors for silent cerebral infarcts in sickle cell anemia: low baseline hemoglobin, sex, and relative high systolic blood pressure. Blood 2012;119(16):3684‐90. - PMC - PubMed
- Eigbire‐Molen O, Darbari DS, Ponisio MR, Milchenko MV, Rodeghier MJ, Casella JF, et al. Progressive loss of brain volume in children with sickle cell anemia: a report from the silent cerebral infarct transfusion trial cohort. Blood 2015;126(23):546. - PubMed
- Glassberg JA, Wang J, Cohen R, Richardson LD, DeBaun MR. Risk factors for increased ED utilization in a multinational cohort of children with sickle cell disease. Academic Emergency Medicine 2012;19(6):664‐72. - PMC - PubMed
- ISRCTN52713285. Silent Cerebral Infarct Multi‐Center Clinical Trial. www.isrctn.com/ISRCTN52713285 24 August 2004. [Protocol/ serial number: U01NS42804]
- Kawadler JM, Clark CA, McKinstry RC, Kirkham FJ. Brain atrophy in paediatric sickle cell anaemia: findings from the silent infarct transfusion (SIT) trial. British Journal of Haematology2016; Vol. Apr 7 [Epub ahead of print]. [DOI: 10.1111/bjh.14039] - DOI - PubMed
- King AA, Rodeghier MJ, Panepinto JA, Strouse JJ, Casella JF, Quinn CT, et al. Silent cerebral infarction, income, and grade retention among students with sickle cell anemia. American Journal of Hematology 2014;89(10):188‐92. - PMC - PubMed
- King, AA, Strouse JJ, Rodeghier MJ, Compas BE, Casella JF, McKinstry RC, et al. Parent education and biologic factors influence on cognition in sickle cell anemia. American Journal of Hematology 2014;89(2):162‐7. - PMC - PubMed
- Kirkham FJ, Darekar E, Kija R, Bhadresha R, McSwiggan J, Cox SE, et al. Brain T2‐weighted signal intensity ratio in children with sickle cell disease with and without stroke. European Journal of Paediatric Neurology 2011;15 Suppl 1:S60. [Abstract no: P07.3]
- Land V, Heijboer H, Fijnvandraat K, DeBaun MR, Casella JF. Transfusions for silent cerebral infarcts in sickle cell anemia. New England Journal of Medicine 2014;371(19):1841‐2. - PubMed
- Liem RI, Liu J, Gordon MO, Vendt BA, McKinstry RC 3rd, Kraut MA, et al. Reproducibility of detecting silent cerebral infarcts in pediatric sickle cell anemia. Journal of Child Neurology 2014;29(12):1685‐91. - PMC - PubMed
- NCT00072761. Silent Cerebral Infarct Transfusion Multi‐Center Clinical Trial (SIT). clinicaltrials.gov/show/NCT00072761 Date first received: 10 November 2003.
- Ogunsile FJ, DeBaun MR, Currie K, Rodeghier MJ. Parvovirus B19 infection associated with silent cerebral infarcts: a secondary analysis of the silent cerebral infarct trial cohort. Blood 2015;126(23):3410. - PubMed
- Quinn CT, McKinstry RC, Dowling MM, Ball WS, Kraut MA, Casella JF, et al. Acute silent cerebral ischemia occurs more frequently than silent cerebral infarction in children with sickle cell anemia. Blood 2010;116(21):268.
- Roberts DO, Covert B, Rodeghier M, Parmar N, DeBaun MR, Thompson AA, et al. Randomization is not associated with socio‐economic and demographic factors in a multi‐center clinical trial of children with sickle cell anemia. Pediatric Blood & Cancer 2014;61(9):1529‐35. - PMC - PubMed
- Thangarajh M, Yang G, Fuchs D, Ponisio MR, McKinstry RC, Jaju A, et al. Magnetic resonance angiography‐defined intracranial vasculopathy is associated with silent cerebral infarcts and glucose‐6‐phosphate dehydrogenase mutation in children with sickle cell anaemia. British Journal of Haematology 2012;159(3):352‐9. - PMC - PubMed
- Vendt BA, McKinstry RC, Ball WS, Kraut MA, Prior FW, Barton B, et al. Silent cerebral infarct transfusion (SIT) trial imaging core: application of novel imaging information technology for rapid and central review of MRI of the brain. Journal of Digital Imaging 2009;22(3):326‐43. - PMC - PubMed
- Wolf RB, Saville BR, Roberts DO, Fissell RB, Kassim AA, Airewele G, et al. Factors associated with growth and blood pressure patterns in children with sickle cell anemia: Silent Cerebral Infarct Multi‐Center Clinical Trial cohort. American Journal of Hematology 2015;90(1):2‐7. - PubMed
- Land V, Heijboer H, Fijnvandraat K, DeBaun MR, Casella JF. Transfusions for silent cerebral infarcts in sickle cell anemia. New England journal of medicine 2014;371(19):1841‐2. - PubMed
-
- Abboud M, Cure J, Gallagher D, Berman B, Hsu L, Wang W, et al. Magnetic resonance angiography (MRA) in children with abnormal transcranial doppler (TCD) velocities in the 'STOP' study. Blood 1999;94(10 Suppl 1):645a. - PubMed
- Abboud MR, Cure J, Gallagher D, Berman B, Hsu L, Wang W, et al. Magnetic resonance angiography in children with abnormal TCD in the STOP study. National Sickle Cell Disease Program Annual Meeting Conference Proceedings; 1999 Mar;. 1999:49.
- Adamkiewicz T, Abboud M, Barredo J, Cavalier ME, Peterson J, Rackoff B, et al. Serum ferritin in children with SCD on chronic transfusion: correlation with serum alanine aminotransferase and liver iron (STOP/STOP II liver iron ancillary study). 35th Anniversary Convention of the National Sickle Cell Disease Program and the Sickle Cell Disease Association of America; 2007 Sep 17‐22; Washington DC. 2007:316.
- Adamkiewicz T, Abboud MR, Barredo JC, Kirby‐Allen M, Alvarez OA, Casella JF, et al. Serum ferritin in children with sickle cell disease on chronic transfusion: measure of iron overload or end organ injury? STOP/ STOP II liver iron ancillary study. Blood2006; Vol. 108, issue 11. [Abstract no: 791]
- Adams R, Brambilla D, McKie V, Files B, Vichinsky E, Abboud M, et al. Stroke prevention in sickle cell disease (STOP): final results. Blood 2000;96(11 Pt 1):10a.
- Adams R, McKie V, Files B, Vichinsky E, Abboud M, Hsu L, et al. Stroke prevention in sickle cell disease (STOP): Results of transcranial doppler ultrasound screening stroke risk. Stroke; a Journal of Cerebral Circulation 1998;29(1):308.
- Adams RJ. Lessons from the stroke prevention trial in sickle cell anemia (STOP) study. Journal of Child Neurology 2000;15(5):344‐9. - PubMed
- Adams RJ, Brambilla D. Stroke prevention in sickle cell anaemia: the 'STOP' trial. 22nd International Joint Conference on Stroke and Cerebral cCrculation; 1997 Feb 6‐8; Anaheim, California. 1997.
- Adams RJ, Brambilla D. Stroke prevention trial in sickle cell anemia: STOP. National Sickle Cell Disease Program Annual Meeting Conference Proceedings; 1996 Mar. 1996:19.
- Adams RJ, Brambilla D, McKie V, Files B, Vichinsky E, Abboud M, et al. Risk of stroke in children with sickle cell disease and abnormal transcranial doppler ultrasound (TCD). Blood 1999;94(10 Suppl 1):419a.
- Adams RJ, Brambilla D, McKie V, Files B, Vichinsky E, Abboud M, et al. Stroke prevention in sickle cell disease (STOP): follow up after closure of the trial. Blood 1998;92(10 Suppl 1):527a.
- Adams RJ, Brambilla D, McKie VC, Files B, Vichinsky E, Abboud M, et al. Stroke prevention in sickle cell disease (STOP): baseline characteristics of trial patients. Blood 1997;90(10 Suppl 1):123a.
- Adams RJ, Brambilla D, Miller ST. Optimizing primary stroke prevention in children with sickle cell. 28th Annual Meeting of the National Sickle Cell Disease Program; 2005 April 9‐13; Cincinnati, Ohio.. 2005:3.
- Adams RJ, Brambilla D, Vichinsky E, Abboud M, Pegelow C, Carl EM, et al. Risk of stroke and conversion to abnormal TCD in children screened with transcranial doppler (TCD) during the STOP study. National Sickle Cell Disease Program Annual Meeting Conference Proceedings; 2002 Sept. 2002:3.
- Adams RJ, Brambilla DJ, McKie V, File B, Vichinsky E, Abboud M. Stroke prevention in sickle cell disease (STOP): Baseline characteristics of trial patients. National Sickle Cell Disease Program Annual Meeting Conference Proceedings; 1997 Sept. 1997:41.
- Adams RJ, Brambilla DJ, STOP II Investigators. The study design of optimizing primary stroke prevention in children with sickle cell anemia. 27th Annual Meeting of the National Sickle Cell Disease Program; 2004 Apr 18‐21; Los Angeles, California. 2004:174.
- Adams RJ, Brambilla DJ, Vichinsky E, Abboud M, Pegelow C, Carl EM, et al. Stroke prevention trial in sickle cell anemia (STOP Study): risk of stroke in 1933 children screened with transcranial doppler (TCD). National Sickle Cell Disease Program Annual Meeting Conference Proceedings; 1999 Mar. 1999:54.
- Adams RJ, Virgil CM, Brambilla D, Carl E, Gallagher D, Nichols FT, et al. Stroke prevention trial in sickle cell anemia. Controlled Clinical Trials 1998;19(1):110‐29. - PubMed
- Files B, Brambilla D, Kutlar A, Miller S, Pegelow C, Vichinsky E, et al. A randomized trial of chronic transfusion to prevent stroke in sickle cell anemia: changes in ferritin with chronic transfusion. Blood 1998;92(10 Suppl 1):528a.
- Gates A, Rogers MA, Puczynski M. Stroke prevention trial in sickle cell anemia: comments on effects of chronic transfusion on pain. Journal of Pediatrics 2002;141(5):742‐3. - PubMed
- Hyacinth HI, Gee BE, Voeks JH, Adams RJ, Hibbert J. High frequency of RBC transfusions in the STOP study was associated with reduction in serum biomarkers of neurodegeneration, vascular remodeling and inflammation. Blood2012; Vol. 120, issue 21. [Abstract no: 244]
- Kutlar A, Harbi J, Jackson B, Holley L, Gallagher D, Clair B, et al. Laboratory parameters in patients randomized in the STOP study and their modification by transfusion. Blood 2000;96(11 Pt 2):18b‐9b.
- Kutlar A, Harbin J, Jackson BB, Holley LGD, Clair B, Brambilla D, et al. Baseline laboratory parameters in patients randomized to the STOP study and their modification by transfusion. National Sickle Cell Disease Program Annual Meeting Conference Proceedings; 2000 Apr. 2000:82a.
- Kwiatkowski JL, Granger S, Brambilla D, Brown RC, Miller S, Adams RJ, et al. Elevated blood flow velocity in the anterior cerebral artery and stroke risk in sickle cell disease: extended analysis from the STOP trial. British Journal of Haematology 2006;134(3):333‐9. - PubMed
- Kwiatkowski JL, Morales K, Brambilla DJ, Files B, Adamkiewicz T, Adams RJ. Long‐term follow‐up of transcranial doppler ultrasonography in children with sickle cell disease: Results of the STOP and STOP II patient cohorts. Blood 2002;100(11 Pt 1):663a.
- Lee MT, Piomelli S, Granger S, Miller ST, Harkness S, Brambilla DJ, et al. Stroke prevention trial in sickle cell anemia (STOP): extended follow‐up and final results. Blood 2006;108(3):847‐52. - PMC - PubMed
- Lee SB, Ramsingh D, Kutlar A, Holley L, McKie VC, Adams RJ. C‐reactive protein in children with sickle cell disease at risk for stroke in the STOP study. Stroke; a Journal of Cerebral Circulation 2002;33(1):373.
- Lezcano NE, Odo N, Kutlar A, Brambilla D, Adams RJ. Transfusion lowers plasma‐free hemoglobin in children with sickle cell disease at risk for stroke. Stroke; a Journal of Cerebral Circulation 2006;37(2):695‐6. - PubMed
- Miller S, Wright E, Abbou M, Berman B, Files B, Scher C, et al. Impact of chronic transfusion on non‐neurological events during the stroke prevention trial in sickle cell anemia (STOP). Pediatric Research 2000;47(4 Pt 2):251A.
- Miller S, Wright E, Abboud M, Berman B, Files B, Scher C, et al. Impact of chronic transfusion on non‐neurological events during the stroke prevention trial in sickle cell anemia (STOP). National Sickle Cell Disease Program Annual Meeting Conference Proceedings; 2000 Apr. 2000:61a.
- NCT00000592. Stroke Prevention in Sickle Cell Anemia (STOP 1). clinicaltrials.gov/ct2/show/NCT00000592 Date first received: 27 October 1999.
- National Heart, Lung, and Blood Institute (NHLBI). Clinical alert: periodic transfusions lower stroke risk in children with sickle cell anemia. https://www.nlm.nih.gov/databases/alerts/sickle97.html (accessed prior to 09 February 2017):www.nlm.nih.gov/databases/alerts/sickle97.html.
- Olivieri N, Brambilla D, McKie V, Piomelli S, Kutlar A, Files B, et al. Changes in cerebral blood flow velocities during chronic transfusion therapy to prevent stroke in sickle cell disease. Blood 2000;96(11 Pt 1):486a.
- Pegelow C, Adams R, Hsu L, McKie V, Wang W, Zimmerman R, et al. Children with silent infarct and elevated transcranial doppler ultrasonography velocity are at increased risk of subsequent infarctive events. National Sickle Cell Disease Program Annual Meeting Conference Proceedings; 1999 Mar. 1999:137.
- Pegelow CH, Wang W, Granger S, Hsu LL, Vichinsky E, Moser FG, et al. Silent infarcts in children with sickle cell anemia and abnormal cerebral artery velocity. Archives of Neurology 2001;58(12):2017‐21. - PubMed
- Sarnaik SA. Prevention of stroke by transfusions in children with sickle cell anemia. New England Journal of Medicine 1998;339:1477‐8. - PubMed
- Vichinsky E, Luban E, Wright E, Olivieri N, Driscoll C, Pegelow, et al. Prospective red cell phenotype matching in STOP ‐ a multi‐centre transfusion trial. National Sickle Cell Disease Program Annual Meeting Conference Proceedings; 1999 Mar. 1999:163.
- Vichinsky E, Luban N, Wright E, Olivieri N, Driscoll C, Pegelow C, et al. Prospective red cell phenotype matching in STOP ‐ a multi‐center transfusion trial. Blood 1998;92(10 Suppl 1):528a.
- Vichinsky E, Luban NL, Wright E, Olivieri N, Driscoll C, Pegelow CH, et al. Prospective RBC phenotype matching in a stroke‐prevention trial in sickle cell anemia: a multicenter transfusion trial. Transfusion 2001;41(9):1086‐92. - PubMed
- Wang W, Morales K, Olivier N, Styles L, Scher C, Adams R, et al. Effect of chronic transfusion on growth in children with sickle cell anemia: results of the STOP trial. National Sickle Cell Disease Program Annual Meeting Conference Proceedings; 2002 Sept. 2002:100.
-
- Abboud MR, Yim E, Adams RJ. The progression and development of silent infarcts in children with sickle cell anemia is prevented by chronic transfusions and is unrelated to level of hemolysis. Blood2008; Vol. 12. [Abstract no: 712]
- Abboud MR, Yim E, Musallam KM, Adams RJ. Discontinuing prophylactic transfusions increases the risk of silent brain infarction in children with sickle cell disease: data from STOP II. Blood 2011;118(4):894‐8. - PMC - PubMed
- Adamkiewicz T. Transcranial doppler measures in patients with sickle cell disease at high risk for stroke and receiving hydroxyurea: the HyRetro ancillary study. 52nd Ash Annual Meeting and Exposition; 2010 Dec 4‐7; Orlando, Florida. 2010. [Abstract no: 1620]
- Adams R, Fullerton H, Kwiatkowski, Voeks J. Development of high risk TCD in sickle cell disease. Stroke; a Journal of Cerebral Circulation2015; Vol. 46, issue Suppl 1. [Abstract no: 17]
- Adams RJ, Brambilla D. Discontinuing prophylactic transfusions used to prevent stroke in sickle cell disease. New England Journal of Medicine 2005;353(26):2769‐78. - PubMed
- Adams RJ, Lackland DT, Brown L, Brown D, Voeks J, Fullerton H, et al. Transcranial doppler re‐screening of subjects who participated in STOP and STOP II. American Journal of Hematology 2016;91(12):1191‐4. - PMC - PubMed
- Brown C, Miller S, Kwiatkowski J, Brambilla D, Adams R. Optimizing primary stroke prevention in sickle cell anemia (STOP 2): an argument for prolonged transfusion?. 29th Annual Meeting of the National Sickle Cell Disease Program; 2006 Apr 8‐12; Memphis, USA.. 2006:78.
- Kanter J, Kwiatkowski J, Fullerton HJ, Voeks J, Debenham E, Brown LJ, Adams RJ. Impact of TCD screening protocol on the incidence of hemorrhagic stroke in children and young adults with sickle cell disease. Blood 2015;126(23):3402.
- Kwiatkowski JL, Kanter J, Fullerton HJ, Voeks J, Debenham E, Brown DG, et al. Ischemic stroke in children and young adults with sickle cell disease (SCD) in the post‐STOP era. Blood 2015;126(23):68. - PubMed
- NCT00006182. Stroke prevention in sickle cell anemia (STOP 2). clinicaltrials.gov/show/NCT00006182 Date first received: 21 August 2000.
- Sayer G, Bowman L, Clair B, Cail A, Blanchard B, Natrajan K, et al. Long term outcome of patients enrolled into STOP and STOP II trials: a single center experience. Blood2012; Vol. 120, issue 21. [Abstract no: 3219]
-
- Aygun B, Mortier NA, Kesler K, Lockhart A, Schultz WH, Cohen A, et al. Therapeutic phlebotomy is safe in children with sickle cell anaemia and can be effective treatment for transfusional iron overload. British Journal of Haematology 2015;169(2):262‐6. - PMC - PubMed
- Aygun B, Mortier NA, Kesler K, Schultz WH, Alvarez OA, Rogers ZR, et al. Therapeutic phlebotomy in children with sickle cell anemia, stroke, and iron overload: the SWiTCH experience. 53rd Ash Annual Meeting and Exposition; 2011 Dec 10‐13; San Diego, California.. 2011. [Abstract no: 1044]
- Helton KJ, Adams RJ, Kesler KL, Lockhart A, Aygun B, Driscoll C, et al. Magnetic resonance imaging/angiography and transcranial Doppler velocities in sickle cell anemia: results from the SWiTCH trial. Blood 2014;124(6):891‐8. - PMC - PubMed
- NCT00122980. Stroke with transfusions changing to hydroxyurea (SWiTCH). clinicaltrials.gov/ct2/show/NCT00122980 Date first received: 20 July 2005.
- National Institutes of Health. Stroke prevention study in children with sickle cell anemia, iron overload stopped early. www.nih.gov/news/health/jun2010/nhlbi‐03.htm (accessed prior to 09 February 2017).
- Ware RE, Helms RW. Stroke with transfusions changing to hydroxyurea (SWiTCH): a phase 3 randomised clinical trial for treatment of children with sickle cell anemia. American Journal of Hematology2011; Vol. 86, issue 11. [Abstract no: 844] - PMC - PubMed
- Ware RE, Helms RW. Stroke with transfusions changing to hydroxyurea (SWiTCH): a phase 3 randomized clinical trial for treatment of children with sickle cell anemia, previous stroke, and iron overload. Blood 2010;116(21):367. - PMC - PubMed
- Ware RE, Helms RW, SWiTCH Investigators. Stroke with transfusions changing to hydroxyurea (SWiTCH). Blood 2012;119(17):3925‐32. - PMC - PubMed
- Ware RE, McMurray MA, Schultz WH, Alvarez OA, Aygun B, Cavalier ME, et al. Academic community standards for chronic transfusion therapy in children with sickle cell anemia and stroke. Blood2006; Vol. 108, issue 11. [Abstract no: 1213]
- Ware RE, Schultz W, Yovetich N, Mortier NA, Alvarez O, Hilliard L, et al. Stroke with transfusions changing to hydroxyurea (SWiTCH): a phase III randomized clinical trial for treatment of children with sickle cell anemia, stroke, and iron overload. Pediatric Blood & Cancer 2011;57(6):1011‐7. - PMC - PubMed
-
- Aygun B, Wruck LM, Schultz WH, Mueller BU, Brown C, Luchtman‐Jones L, et al. Chronic transfusion practices for prevention of primary stroke in children with sickle cell anemia and abnormal TCD velocities. American Journal of Hematology 2012;87(4):428‐30. - PubMed
- Helton K, Roberts D, Schultz WH, Davis BR, Kalfa TA, Pressel SL, et al. Effects of Chronic Transfusion Therapy on MRI and MRA in Children with Sickle Cell Anemia at Risk for Primary Stroke: Baseline Imaging from theTwitch Trial. Blood 2014;124(21):4052.
- Imran H, Aygun B, Davis BR, Pressel SL, Schultz WH, Jackson S, et al. Effects of chronic transfusion therapy on transcranial doppler ultrasonography velocities in children with sickle cell anemia at risk for primary stroke: Baseline findings from the Twitch trial. Blood 2014;124(21):87.
- NCT01425307. Transcranial doppler (TCD) with transfusions changing to hydroxyurea [TCD with transfusions changing to hydroxyurea (TWiTCH): a phase III randomized trial to compare standard therapy (erythrocyte transfusions) with alternative therapy (hydroxyurea) for the maintenance of lowered TCD velocities in pediatric subjects with sickle cell anemia and abnormal pre‐treatment TCD velocities]. clinicaltrials.gov/ct2/show/NCT01425307 Date first received: 19 August 2011.
- Ware RE, Davis BR, Schultz WH, Brown C, Aygun B, Sarnaik SA, et al. TCD with transfusions changing to hydroxyurea (TWiTCH): hydroxyurea therapy as an alternative to transfusions for primary stroke prevention in children with sickle cell anemia. Blood 2015;126(23):3.
- Ware RE, Davis BR, Schultz WH, Brown RC, Aygun B, Sarnaik S, et al. Hydroxycarbamide versus chronic transfusion for maintenance of transcranial doppler flow velocities in children with sickle cell anaemia ‐ TCD with Transfusions Changing to Hydroxyurea (TWiTCH): a multicentre, open‐label, phase 3, non‐inferiority trial. Lancet 2016;387(10019):661‐70. - PMC - PubMed
- Wood JC, Cohen AR, Pressel SL, Aygun B, Imran H, Luchtman‐Jones L, et al. Organ iron accumulation in chronically transfused children with sickle cell anaemia: baseline results from the TWiTCH trial.. British Journal of Haematology 2016;172(1):122‐30. - PMC - PubMed
- Wood JC, Pressel S, Rogers ZR, Odame I, Kwiatkowski JL, Lee MT, et al. Liver iron concentration measurements by MRI in chronically transfused children with sickle cell anemia: baseline results from the TWiTCH trial.. American Journal of Hematology 2015;90(9):806‐10. - PMC - PubMed
References to studies excluded from this review
-
- Adams RJ, Carl EM, McKie VC, Odo NA, Kutlar A, Phillips M, Brambilla D. A pilot trial of hydroxyurea to prevent strokes in children with sickle cell anemia. National Sickle Cell Disease Program Annual Meeting Conference Proceedings; 1999 Mar. 1999:53.
-
- Adams RJ, Luden J, Miller S, Wang W, Rees R, Li D, et al. TCD in infants: a report from the Baby Hug study. 28th Annual Meeting of the National Sickle Cell Disease Program; 2005 Apr 9‐13; Cincinnati, Ohio.. 2005:105.
- Alvarez O, Miller ST, Wang WC, Luo Z, McCarville MB, Schwartz GJ, et al. Effect of hydroxyurea treatment on renal function parameters: results from the multi‐center placebo‐controlled BABY HUG clinical trial for infants with sickle cell anemia. Pediatric Blood & Cancer 2012;59(4):668‐74. - PMC - PubMed
- Armstrong FD, Elkin TD, Brown RC, Glass P, Rana S, Casella JF, et al. Developmental function in toddlers with sickle cell anemia. Pediatrics 2013;131(2):e406‐14. - PMC - PubMed
- Kalpatthi R, Thompson B, Lu M, Wang WC, Patel N, Kutlar A, et al. Comparison of hematologic measurements between local and central laboratories: data from the BABY HUG trial. Clinical Biochemistry 2013;46(3):278‐81. - PMC - PubMed
- Lebensburger JD, Miller ST, Howard TH, Casella JF, Brown RC, Lu M, et al. Influence of severity of anemia on clinical findings in infants with sickle cell anemia: analyses from the BABY HUG study. Pediatric Blood & Cancer 2012;59(4):675‐8. - PMC - PubMed
- Lederman HM, Connolly MA, Ware RE, Luchtman‐Jones L, Goldsmith JC. Effects of hydroxyurea (HU) on lymphocyte subsets and the immune response to pneumococcal, measles, mumps and rubella vaccination in the pediatric hydroxyurea phase III clinical trial ‐ BABY HUG. Blood2012; Vol. 120, issue 21. [Abstract no: 243]
- McGann PT, Flanagan JM, Howard TA, Dertinger SD, He J, Kulharya AS, et al. Genotoxicity associated with hydroxyurea exposure in infants with sickle cell anemia: results from the BABY‐HUG Phase III Clinical Trial. Pediatric Blood & Cancer 2012;59(2):254‐7. - PMC - PubMed
- Sheehan VA, Luo Z, Flanagan JM, Howard TA, Thompson BW, Wang WC, et al. Genetic modifiers of sickle cell anemia in the BABY HUG cohort: influence on laboratory and clinical phenotypes. American Journal of Hematology 2013;88(7):571‐6. - PubMed
- Thornburg CD, Files BA, Luo Z, Miller ST, Kalpatthi R, Iyer R, et al. Impact of hydroxyurea on clinical events in the BABY HUG trial. Blood 2012;120(22):4304‐10; quiz 4448. - PMC - PubMed
- Wang WC, Oyeku S, Luo Z, Boulet SL, Miller ST, Casella J, et al. Hydroxyurea is associated with lower costs of care of young children with sickle cell anemia. Pediatrics 2013;132(4):67783. - PMC - PubMed
- Wang WC, Pavlakis SG, Helton KJ, McKinstry R, Casella J, Adams RJ, et al. MRI abnormalities of the brain in one‐year‐old children with sickle cell anemia. Pediatric Blood & Cancer 2008;51(5):643‐6. - PubMed
- Wang WC, Ware RE, Miller ST, Iyer RV, Casella JF, Minniti CP, et al. Hydroxycarbamide in very young children with sickle cell anaemia: a multicentre, randomised, controlled trial (BABY HUG). Lancet 2011;377(9778):1663‐72. - PMC - PubMed
-
- Bernaudin F, Verlhac S, Ducros‐Miralles E, Delatour RP, Dalle JH, Petra E, et al. French national Drepagreffe trial: cognitive performances and neuroimaging at enrollment and after 12 months on transfusion program or transplantation (AP‐HP: NCT 01340404). Blood2015; Vol. 126, issue 23:544.
-
- Bernaudin F, Verlhac S, Ducros ME, Peffault DR, Dalle JH, Paillard C, et al. Trial comparing HSCT vs transfusions in sickle cell anemia (SCA) patients with abnormal cerebral velocities: cerebral vasculopathy outcome at 1 year. Bone Marrow Transplantation 2016;51:S49‐50.
-
- Kawadler JM, Clayden JD, Clark CA, Kirkham FJ. Intelligence quotient in paediatric sickle cell disease: a systematic review and meta‐analysis. Developmental Medicine & Child Neurology 2016;58(7):672‐9. - PubMed
References to studies awaiting assessment
-
- NCT00850018. Examining cognitive function and brain abnormalities in adults with sickle cell disease ‐ pilot intervention study [Neuropsychological dysfunction and neuroimaging abnormalities in neurologically intact adults with sickle cell disease ‐ a pilot intervention study]. clinicaltrials.gov/ct2/show/NCT00850018 Date first received: 20 February 2009.
- Vichinsky E, Neumay L, Gold JI, Weiner MW, Kasten J, Truran D, et al. A Randomized trial of the safety and benefit of transfusion vs. standard care in the prevention of sickle cell‐related complications in adults: a preliminary report from the phase I NHLBI comprehensive sickle cell centers (cscc) study of neuropsychological dysfunction and neuroimaging abnormalities in neurologically intact adult patients with sickle cell disease. Blood 2010;116(21):1321. - PubMed
- Vichinsky E, Neumayr L, Gold JI, Weiner MW, Kasten J, Truran D. A randomised trial of the safety and benefit of transfusion vs. standard care in the prevention of sickle cell‐related complications in adults: a preliminary report from the phase II NHLBI comprehensive sickle cell centers (CSCC) study of neuropsychological dysfunction and neuroimaging abnormalities in neurologically intact adult patients with sickle cell disease. 52nd Ash Annual Meeting and Exposition; 2010 Dec 4‐7; Orlando, Florida.. 2010. [Abstract no: 3221]
References to ongoing studies
-
- NCT01389024. Hydroxyurea to prevent brain injury in sickle cell disease (HUPrevent) [Hydroxyurea to prevent central nervous system (CNS) complications of sickle cell disease in children]. clinicaltrials.gov/ct2/show/NCT01389024 Date first received: 30 June 2011.
Additional references
-
- Adams RJ, McKie VC, Hsu L, Files B, Vichinsky E, Pegelow C, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. New England Journal of Medicine 1998;339(1):5‐11. - PubMed
-
- Adekile AD, Yacoub F, Gupta R, Sinan T, Haider MZ, Habeeb Y, et al. Silent brain infarcts are rare in Kuwaiti children with sickle cell disease and high Hb F. Americal Journal of Hematology 2002;70(3):228‐31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

