Enhanced Tau Aggregation in the Presence of Amyloid β
- PMID: 28500862
- PMCID: PMC5500829
- DOI: 10.1016/j.ajpath.2017.03.011
Enhanced Tau Aggregation in the Presence of Amyloid β
Abstract
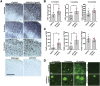
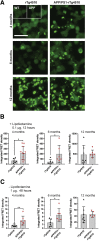
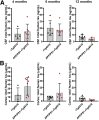
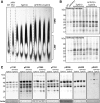
Amyloid plaques and neurofibrillary tangles co-occur in Alzheimer disease, but with different topological and temporal patterns. Whether these two lesions are independent or pathobiologically related is uncertain. For example, amyloid deposition in the neocortex precedes the spread of tau neurofibrillary tangles from the limbic areas to the cortex. We examined the aggregation properties of tau isolated from human cases with early tau pathology (Braak II) with and without plaques. Using a well-established HEK cell biosensor assay, we show that tau from cases with plaques has an enhanced ability to induce tau aggregates compared to tau from cases without plaques. To further explore this effect, we combined mice carrying the APP/PS1 transgene array that develop plaques with rTg4510 mice carrying the P301L mutant human tau transgene that develop extensive tau pathology with age. The resulting APP/PS1-rTg4510 mice had a threefold increase in tau seeding activity over the rTg4510 strain, without change in tau production or extracellular release. Surprisingly, this effect was observed before overt amyloid deposition. The enhancement of tau aggregation was also apparent by an increase in histological measures of tau pathology in young APP/PS1-rTg4510 mice and an increase in high-molecular-weight tau. Overall, these data provide evidence that amyloid β acts to enhance tau pathology by increasing the formation of tau species capable of seeding new aggregates.
Copyright © 2017 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures




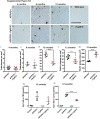
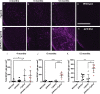
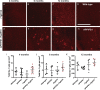
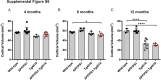
References
-
- Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. - PubMed
-
- Thal D.R., Rüb U., Orantes M., Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. - PubMed
-
- Arriagada P.V., Growdon J.H., Hedley-Whyte E.T., Hyman B.T. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42:631–639. - PubMed
-
- Bierer L.M., Hof P.R., Purohit D.P., Carlin L., Schmeidler J., Davis K.L., Perl D.P. Neocortical neurofibrillary tangles correlate with dementia severity in Alzheimer's disease. Arch Neurol. 1995;52:81–88. - PubMed
-
- Bolmont T., Clavaguera F., Meyer-Luehmann M., Herzig M.C., Radde R., Staufenbiel M., Lewis J., Hutton M., Tolnay M., Jucker M. Induction of tau pathology by intracerebral infusion of amyloid-β-containing brain extract and by amyloid-β deposition in APP × Tau transgenic mice. Am J Pathol. 2007;171:2012–2020. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

