Direct orthotopic implantation of hepatic organoids
- PMID: 28501125
- PMCID: PMC5433258
- DOI: 10.1016/j.jss.2016.12.028
Direct orthotopic implantation of hepatic organoids
Abstract
Background: Liver organoids show potential for development as a tissue replacement therapy for patients with end-stage liver disease, but efficient methods for introducing organoids into host livers have not been established. In this study, we aimed to develop a surgical technique to implant hepatic organoids into the liver and assess their engraftment.
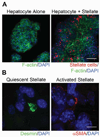
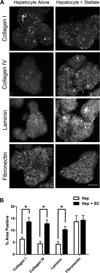
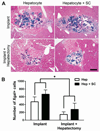
Methods: Donor hepatocytes were isolated from ROSA26 C57BL/6 mice, so that engrafted cells, when implanted into wild-type mice, could be easily identified by X-gal staining. Hepatic organoids were generated by three-dimensional culture in rotating wall vessel bioreactors. We qualitatively and quantitatively compared organoid engraftment to that of single-cell hepatocyte transplants. In addition, we determined the effect of adding stellate cells to hepatocytes to form co-aggregated organoids and the effect of partial hepatectomy of the host liver on organoid engraftment.
Results: Direct orthotopic implantation of hepatic organoids within a hepatotomy site resulted in local engraftment of exogenous hepatocytes with limited durability. Hepatocyte-stellate cell organoids produced more extracellular matrix but did not significantly improve engraftment compared with hepatocyte-alone organoids. Partial hepatectomy of the host liver led to significantly decreased engraftment of organoids. Survival of organoids was limited by the presence of apoptotic hepatocytes within organoids as early as 1 h after implantation. Organoids eventually became necrotic and elicited a chronic inflammatory giant cell reaction similar to a foreign body response.
Conclusions: With additional organoid and host factor optimization, direct orthotopic implantation of hepatic organoids may be an approach to introduce large numbers of exogenous hepatocytes into recipient livers.
Keywords: Liver organoids; Three-dimensional culture; Tissue engineering; Transplantation.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no conflicts to disclose.
Figures



References
-
- Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345:1247125. - PubMed
-
- 12/2/2014 ed: Centers for Disease Control and Prevention. 2015. Deaths, Percent of Total Deaths, and Death Rates for the 15 Leading Causes of Death: United States and Each State, 1999–2013.
-
- Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481–484. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

