MicroRNAs in injury and repair
- PMID: 28501916
- PMCID: PMC5843992
- DOI: 10.1007/s00204-017-1974-1
MicroRNAs in injury and repair
Abstract
Organ damage and resulting pathologies often involve multiple deregulated pathways. MicroRNAs (miRNAs) are short, non-coding RNAs that regulate a multitude of genes at the post-transcriptional level. Since their discovery over two decades ago, miRNAs have been established as key players in the molecular mechanisms of mammalian biology including the maintenance of normal homeostasis and the regulation of disease pathogenesis. In recent years, there has been substantial progress in innovative techniques to measure miRNAs along with advances in targeted delivery of agents modulating their expression. This has expanded the scope of miRNAs from being important mediators of cell signaling to becoming viable quantitative biomarkers and therapeutic targets. Currently, miRNA therapeutics are in clinical trials for multiple disease areas and vast numbers of patents have been filed for miRNAs involved in various pathological states. In this review, we summarize miRNAs involved in organ injury and repair, specifically with regard to organs that are the most susceptible to injury: the liver, heart and kidney. In addition, we review the current state of knowledge on miRNA biology, miRNA biomarkers and nucleotide-based therapeutics designed to target miRNAs to prevent organ injury and promote repair.
Keywords: Heart; Injury; Kidney; Liver; MicroRNAs; Repair.
Figures


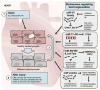

References
-
- Breving K, Esquela-Kerscher A. The complexities of microRNA regulation: mirandering around the rules. The international journal of biochemistry & cell biology. 2010;42(8):1316–1329. - PubMed
-
- Ding XC, Weiler J, Grosshans H. Regulating the regulators: mechanisms controlling the maturation of microRNAs. Trends in biotechnology. 2009;27(1):27–36. - PubMed
-
- Marco A, Ninova M, Griffiths-Jones S. Multiple products from microRNA transcripts. Biochemical Society transactions. 2013;41(4):850–854. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

